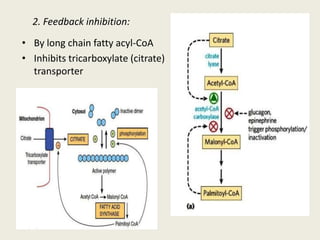
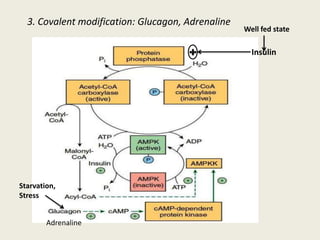
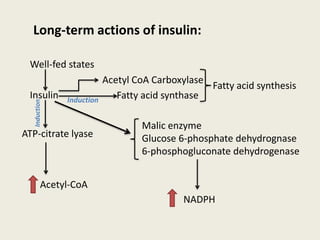
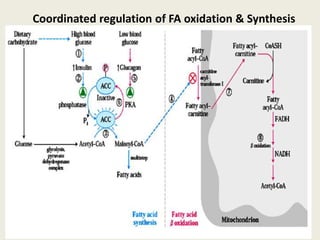
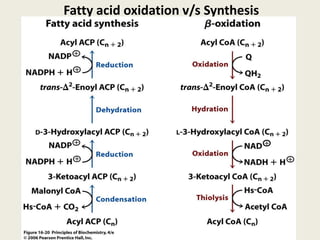
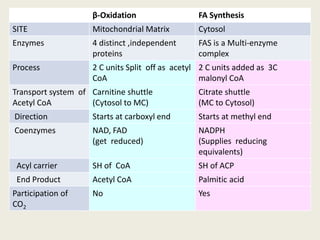
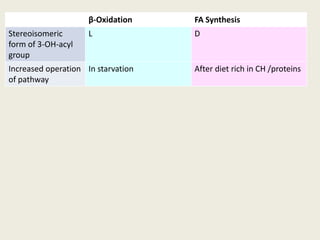
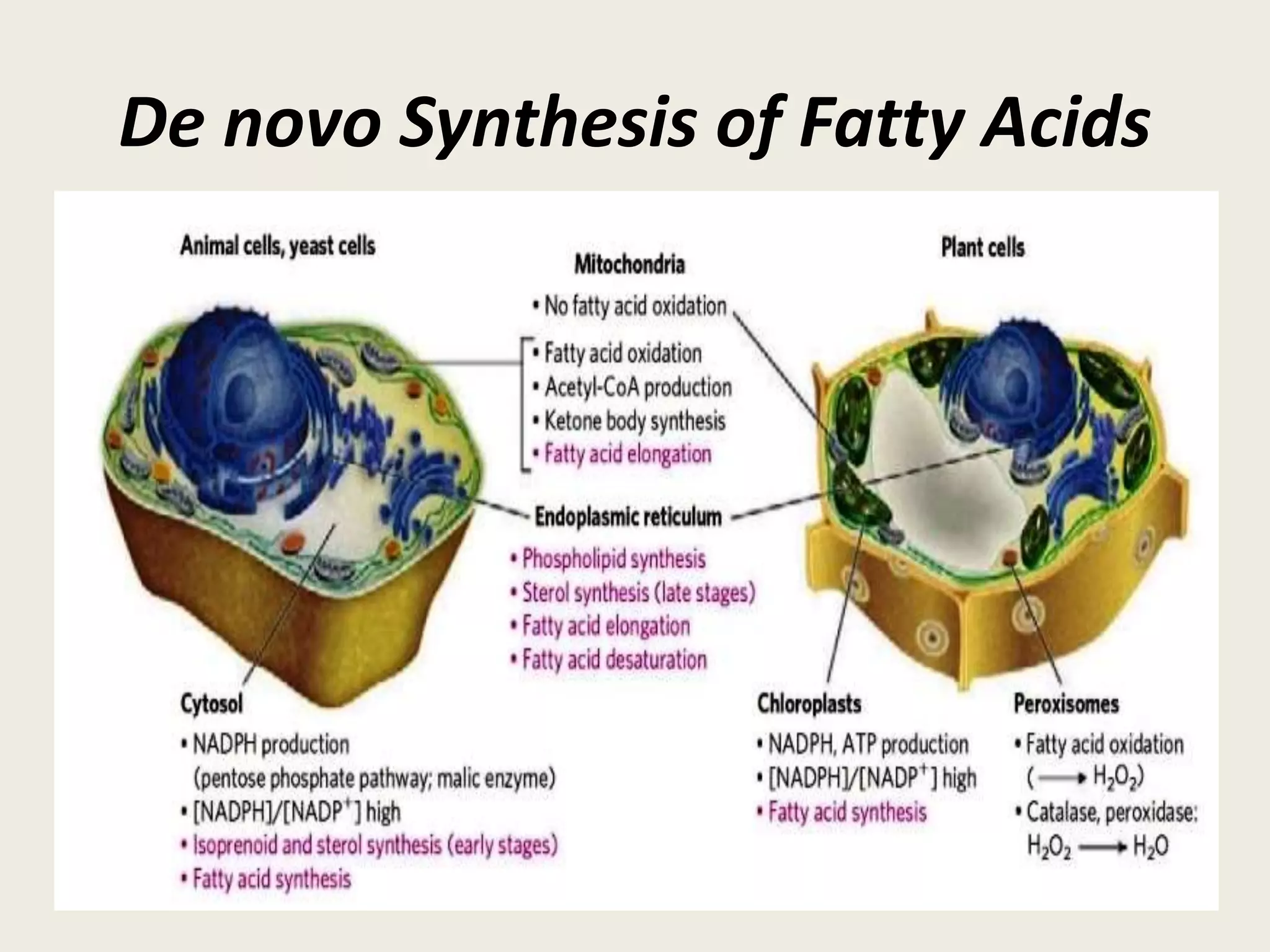
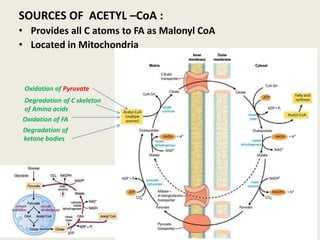
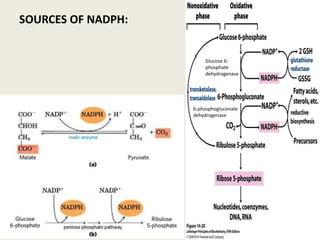
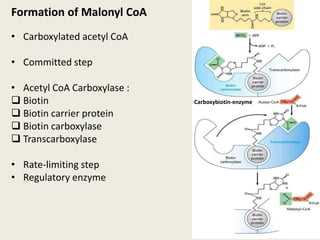
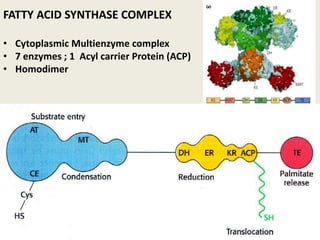
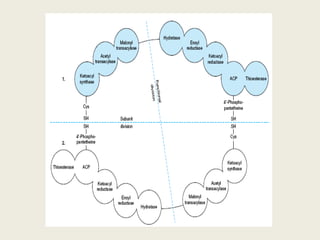
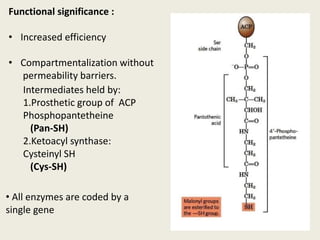
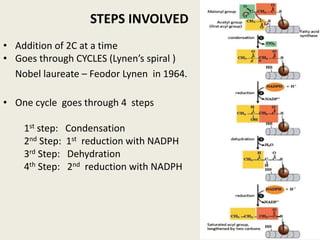
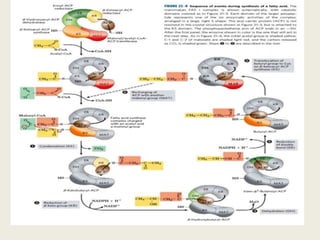
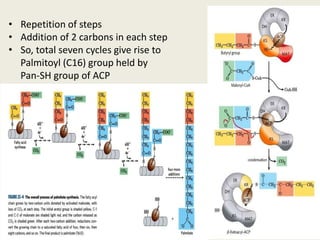
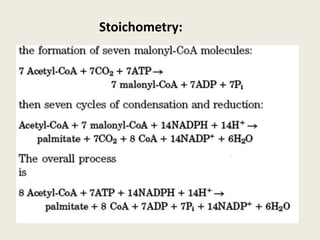
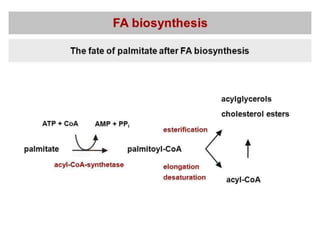
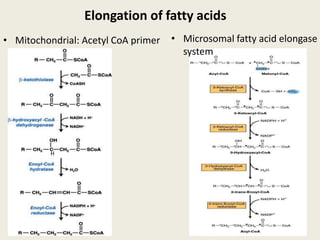
This document summarizes de novo fatty acid synthesis. Fatty acid synthesis occurs in the cytosol using acetyl-CoA and malonyl-CoA as substrates. Acetyl-CoA carboxylase converts acetyl-CoA to malonyl-CoA in a committed step regulated by insulin and glucagon. A fatty acid synthase complex containing seven enzymes then catalyzes the sequential addition of two-carbon units from malonyl-CoA to produce palmitate through cycles of condensation, reduction, dehydration, and reduction. Elongation and desaturation of fatty acids are accomplished by accessory enzyme systems to produce a variety of fatty acid products.





![Regulation of Acetyl CoA Carboxylase:
1. Allosteric activation
[phospho]
[dephospho]
• Carbohydrate oxidation
(well fed state)
Citrate](https://image.slidesharecdn.com/fattyacidsynthesisupload-170721145924/85/Fatty-acid-synthesis-19-320.jpg)