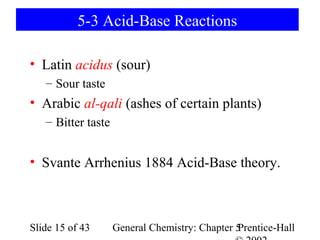
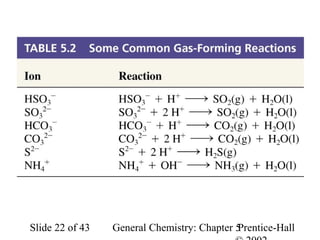
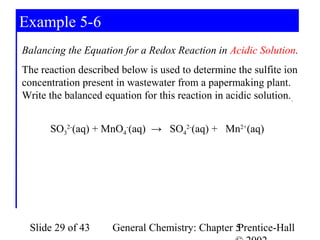
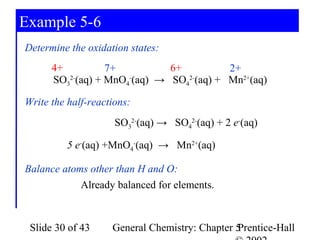
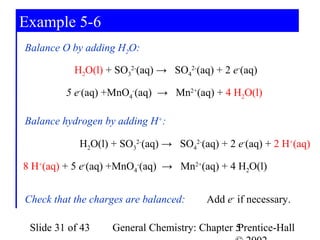
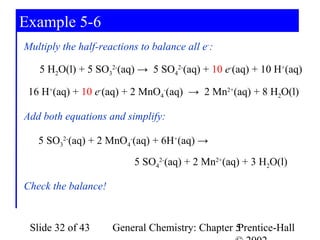
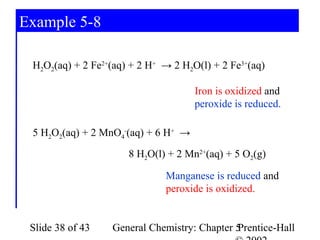
Here are the steps to balance this redox reaction:
1) Write the half-reactions:
SO32- oxidation: SO32- → SO42- + 2e-
MnO4- reduction: MnO4- + 8H+ + 5e- → Mn2+ + 4H2O
2) Balance all atoms except oxygen and hydrogen:
SO32- → SO42- + 2e-
MnO4- + 5e- → Mn2+
3) Balance oxygen atoms:
SO32- → SO42- + 2e-
MnO4- + 8H+ + 5e- → Mn2+ + 4H2O
4) Balance charges:



![Notation for Concentration
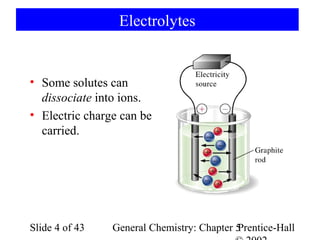
MgCl2(s) → Mg2+(aq) + 2 Cl-(aq)
In 0.0050 M MgCl2:
Stoichiometry is important.
[Mg2+] = 0.0050 M [Cl-] = 0.0100 M [MgCl2] = 0 M
Slide 7 of 43 General Chemistry: Chapter 5
Prentice-Hall](https://image.slidesharecdn.com/ch05-121227103346-phpapp02/85/Ch05-7-320.jpg)
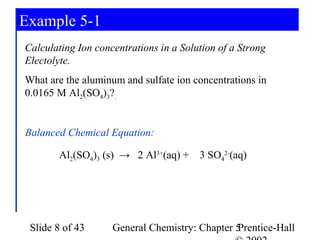
![Example 5-1
Aluminum Concentration:
0.0165 mol Al2(SO4)3 2 mol Al3+
[Al] = × = 0.0330 M Al3+
1L 1 mol Al2(SO4)3
Sulfate Concentration:
0.0165 mol Al2(SO4)3 3 mol SO42-
[SO4 ] =
2-
× = 0.0495 M SO42-
1L 1 mol Al2(SO4)3
Slide 9 of 43 General Chemistry: Chapter 5
Prentice-Hall](https://image.slidesharecdn.com/ch05-121227103346-phpapp02/85/Ch05-9-320.jpg)

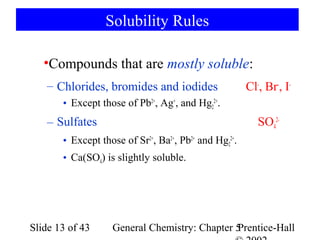




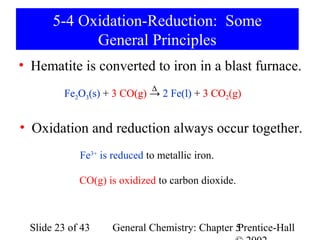
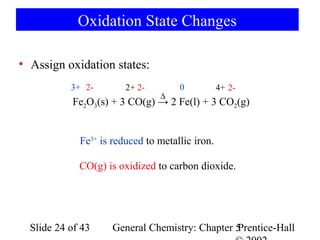
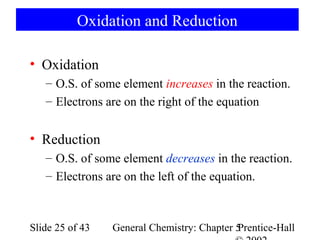
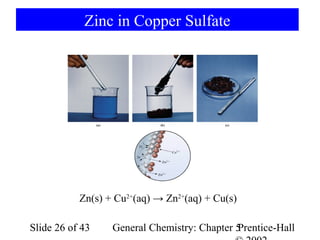
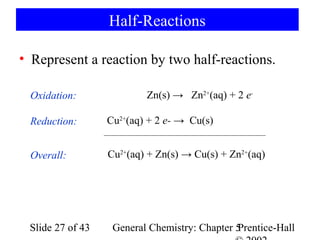





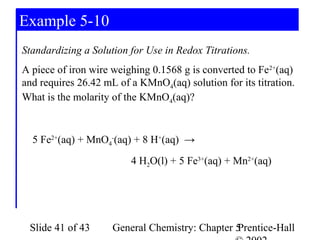
![Example 5-10
5 Fe2+(aq) + MnO4-(aq) + 8 H+(aq) → 4 H2O(l) + 5 Fe3+(aq) + Mn2+(aq)
Determine KMnO4 consumed in the reaction:
1 mol Fe 1 mol Fe 2 +
nH 2O = 0.1568 g Fe × × ×
55.847 g Fe 1 mol Fe
−
1 mol MnO4 1 mol KMnO4
× −
= 5.615 × 10 − 4 mol KMnO4
5 mol Fe 2 + 1 mol MnO4
Determine the concentration:
5.615 × 10 −4 mol KMnO4
[ KMnO4 ] = = 0.02140 M KMnO4
0.02624 L
Slide 42 of 43 General Chemistry: Chapter 5
Prentice-Hall](https://image.slidesharecdn.com/ch05-121227103346-phpapp02/85/Ch05-42-320.jpg)