Embed presentation
Downloaded 221 times

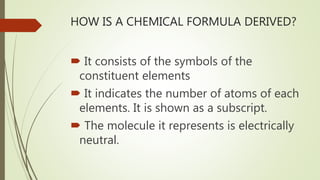


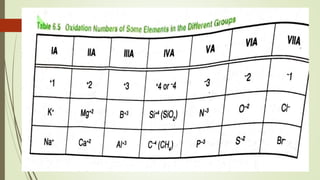
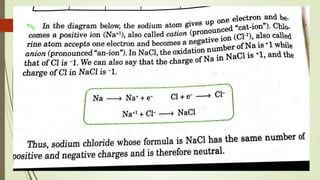

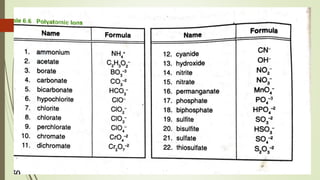


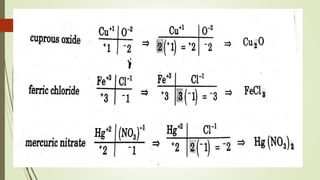
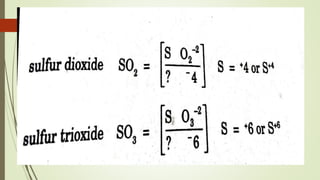


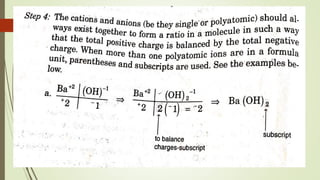
A chemical formula consists of the symbols of the constituent elements, with subscripts indicating the number of atoms of each element. It represents an electrically neutral molecule. Oxidation numbers are assigned to atoms based on whether they gain, lose, or share electrons, and help describe chemical compounds - atoms that lose electrons are assigned a positive number, while atoms that gain electrons are assigned a negative number.














