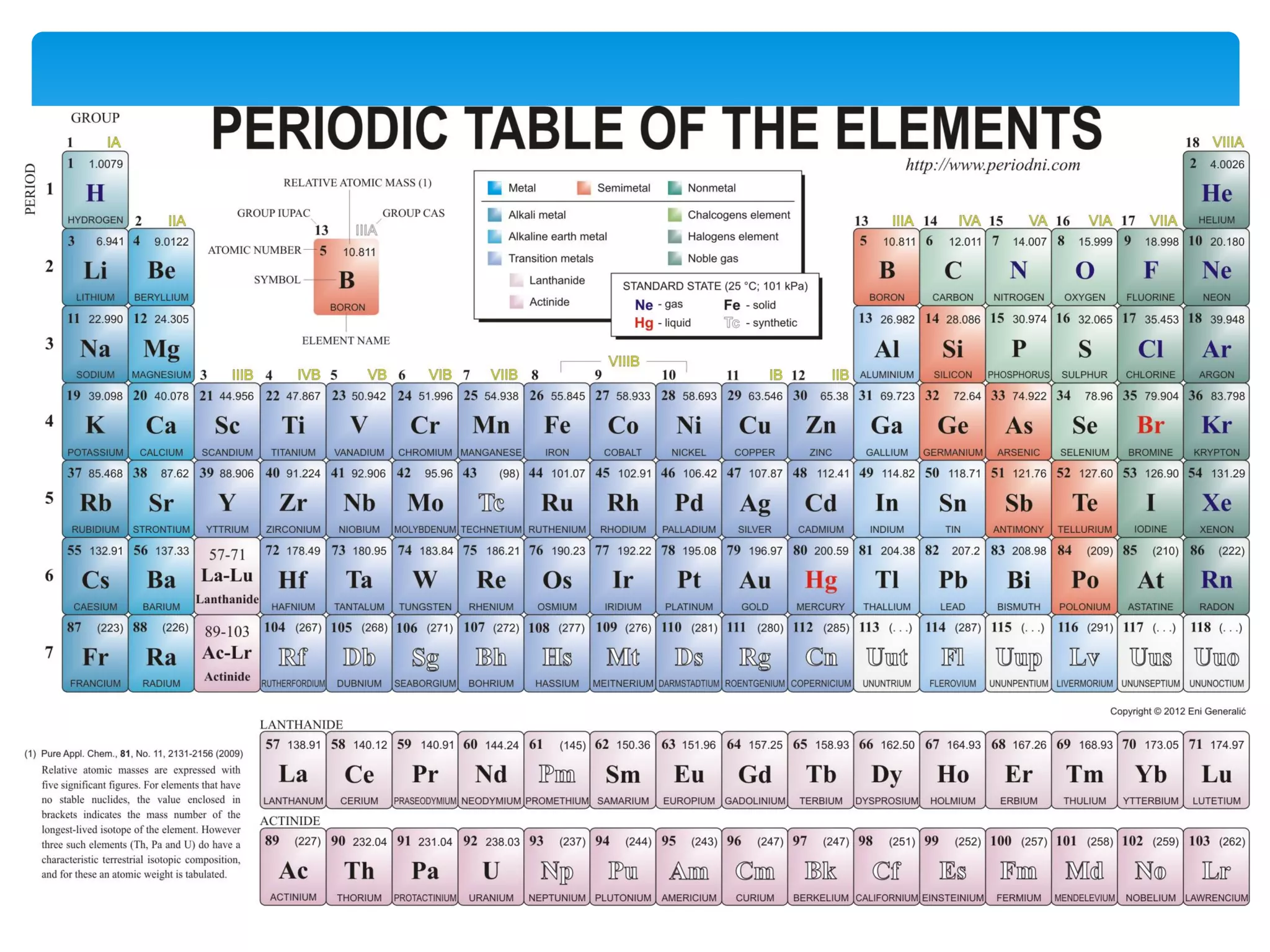
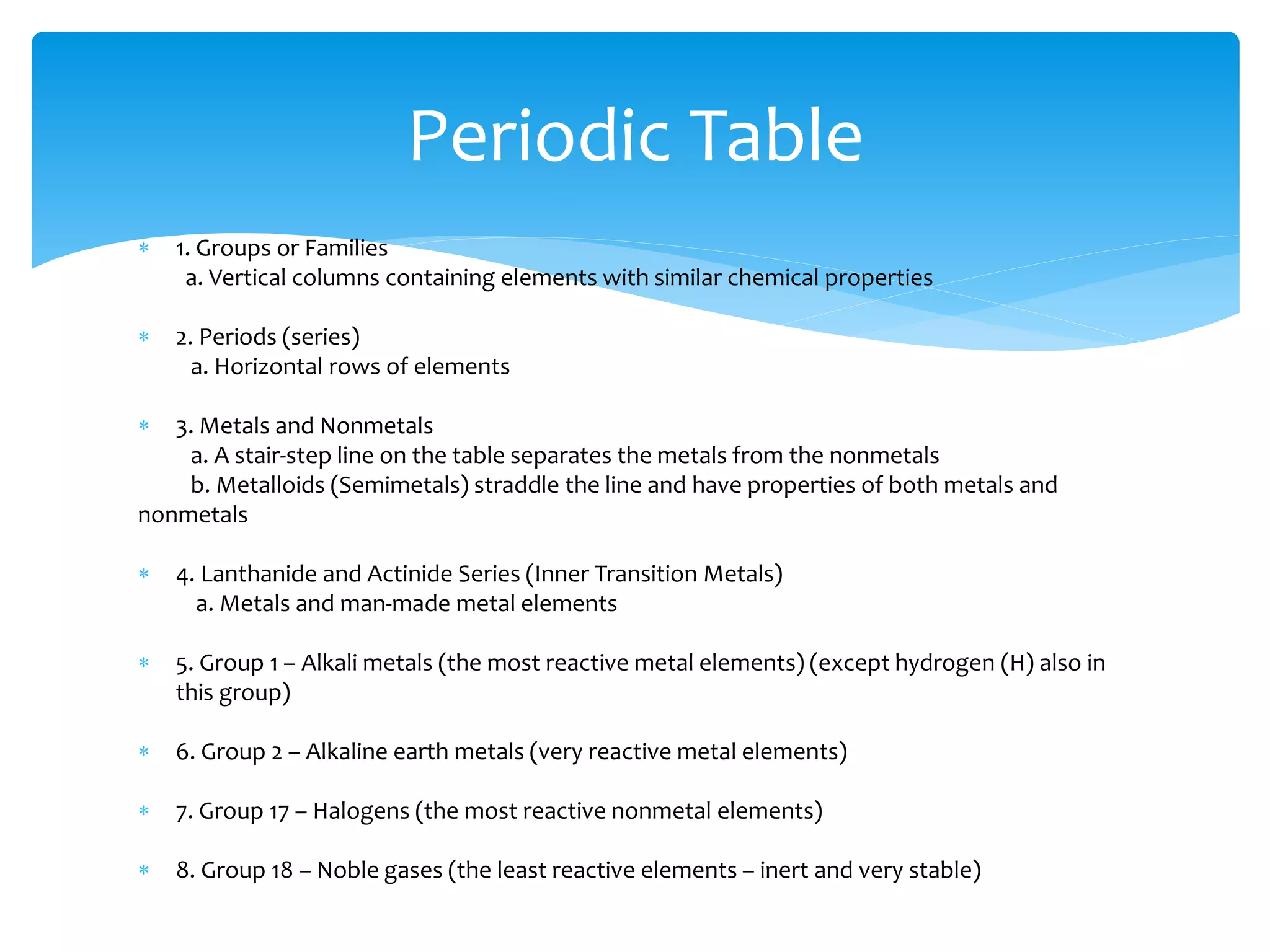
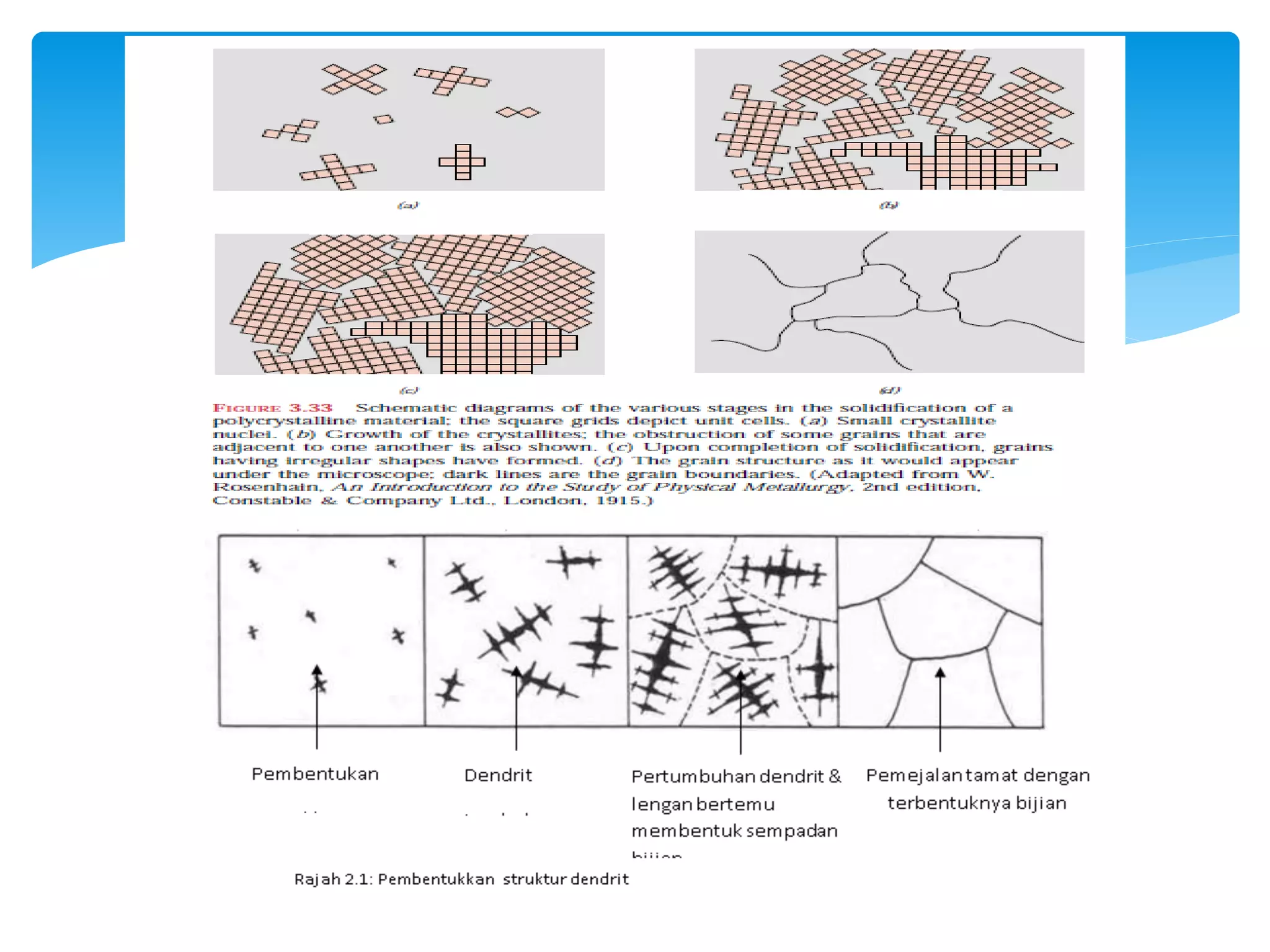
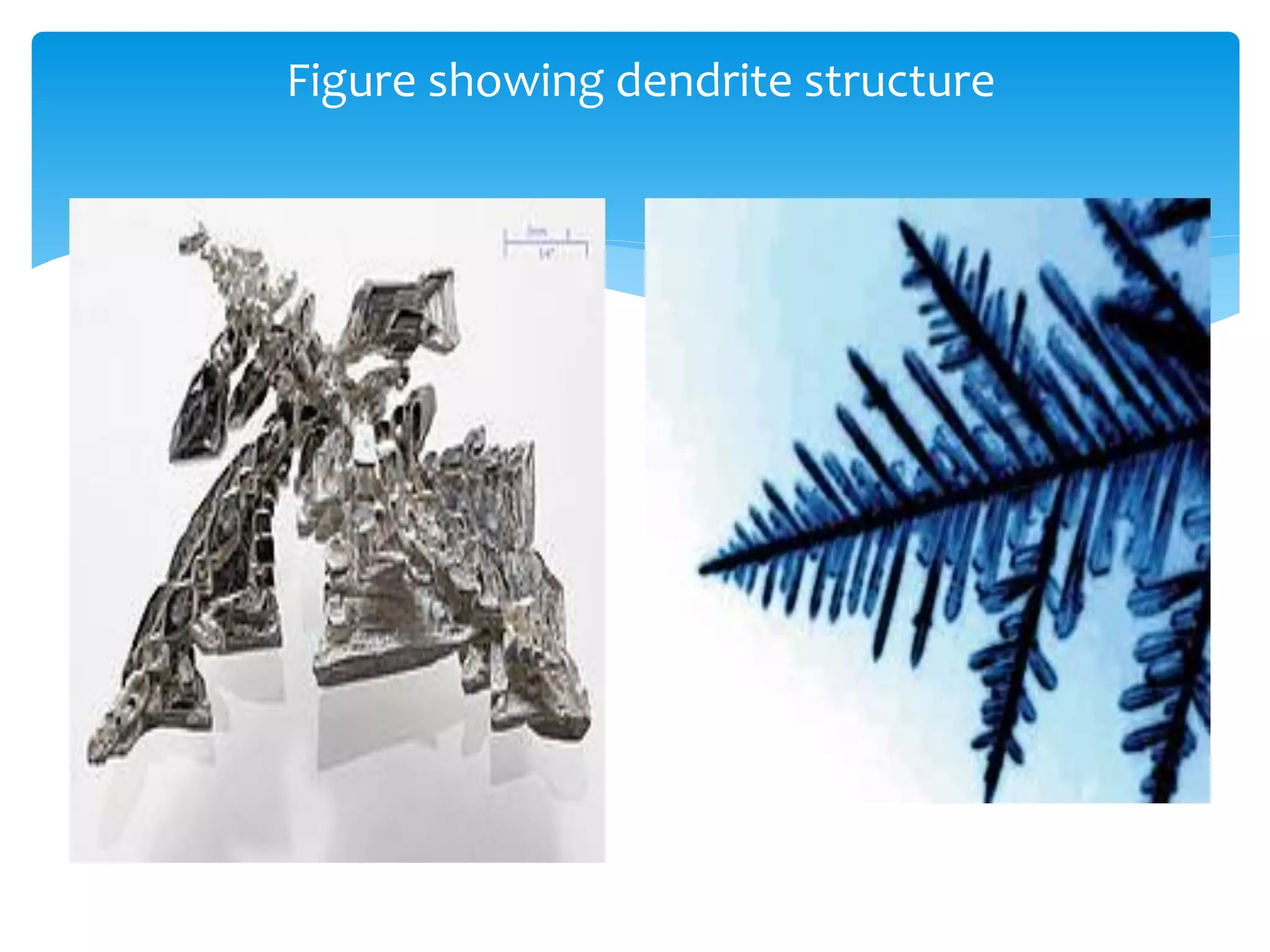
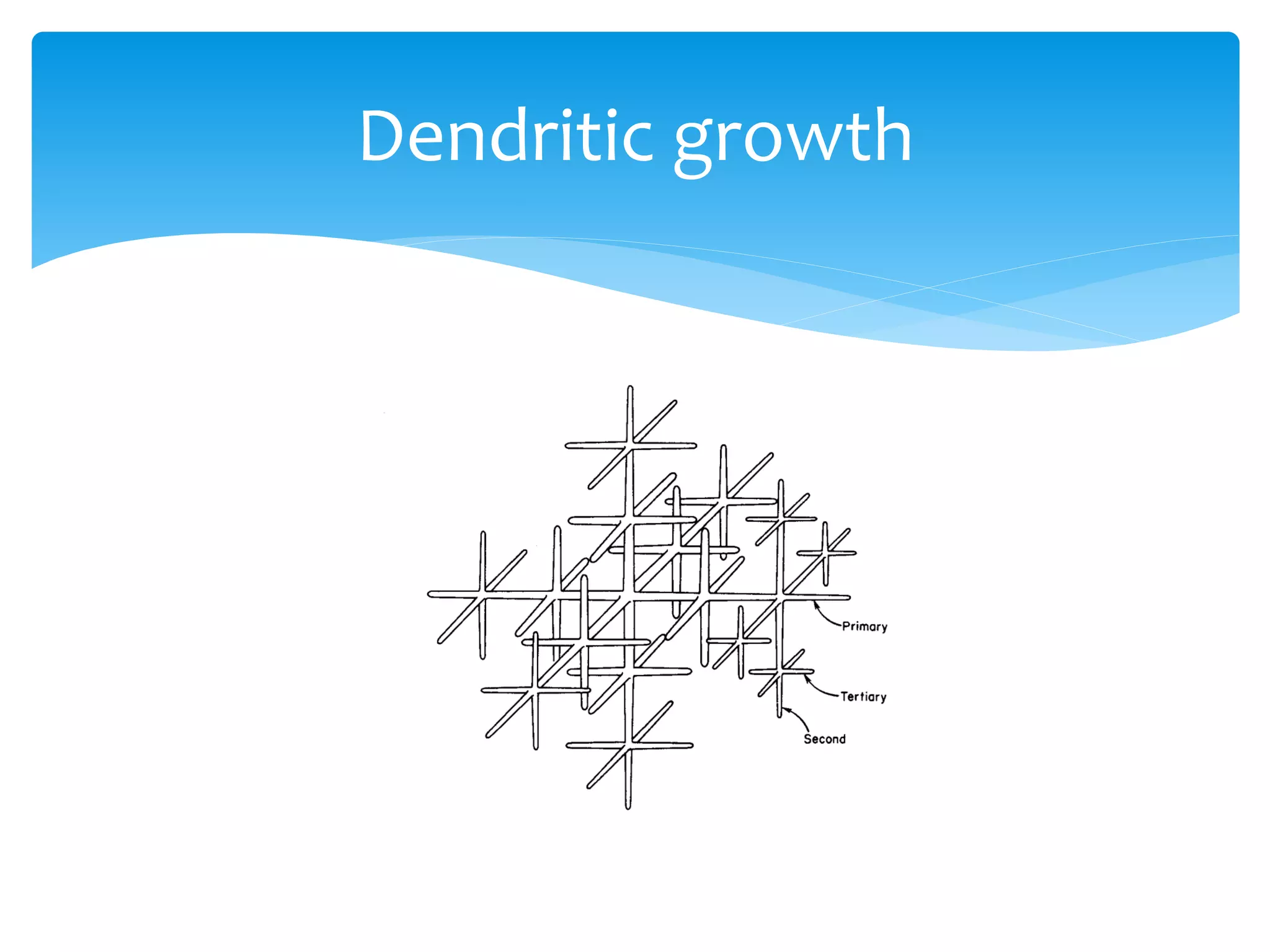
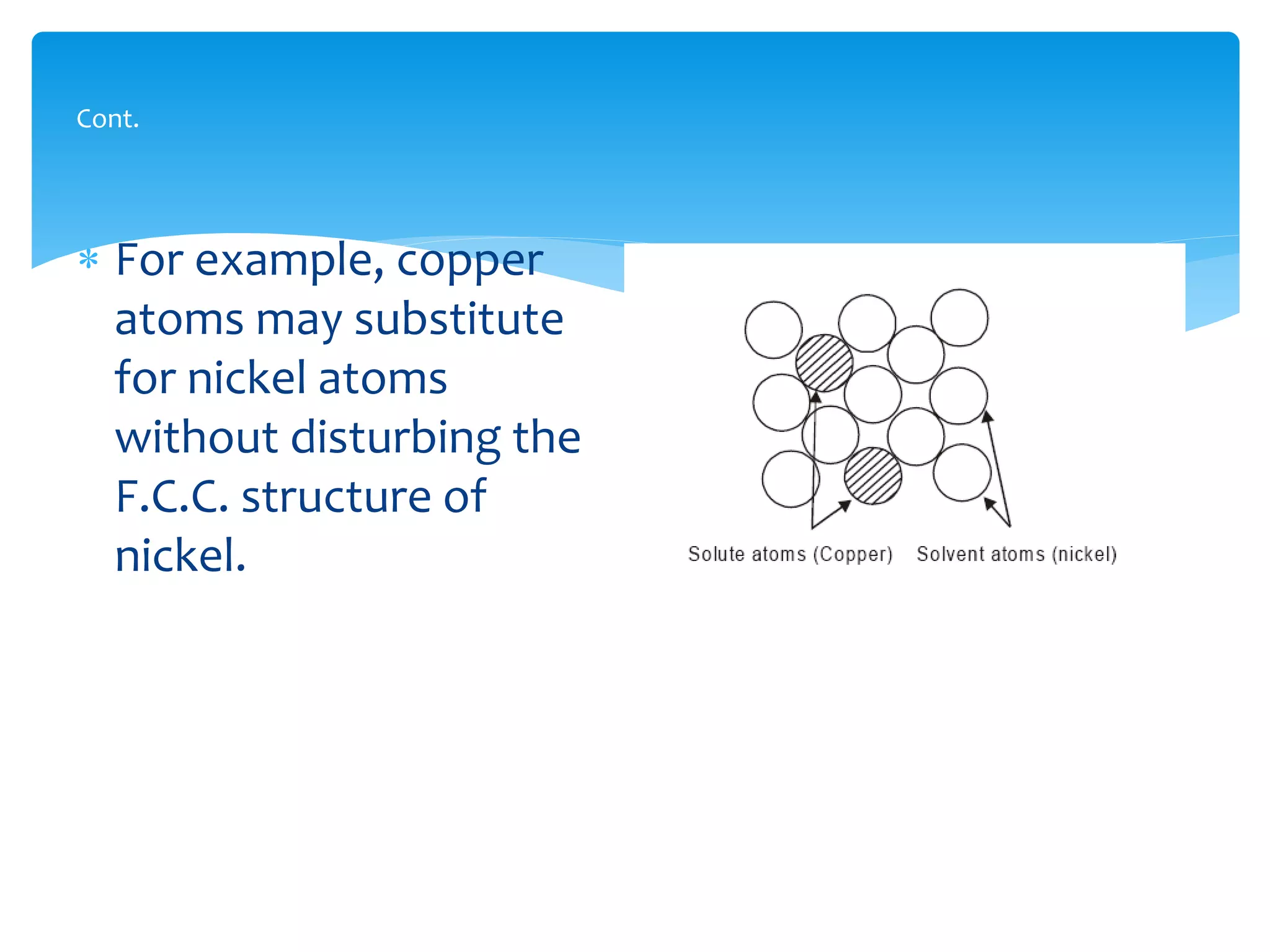
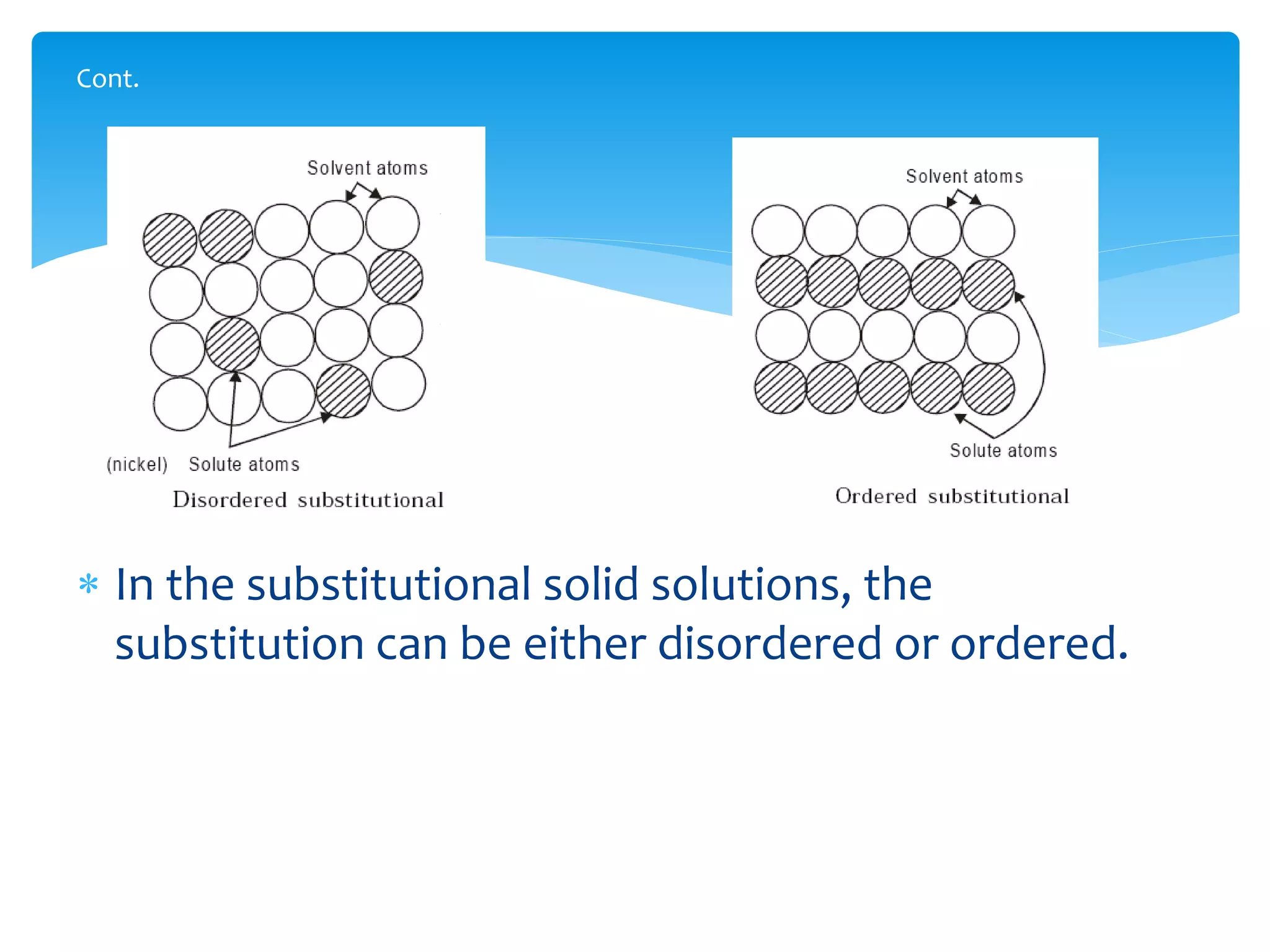
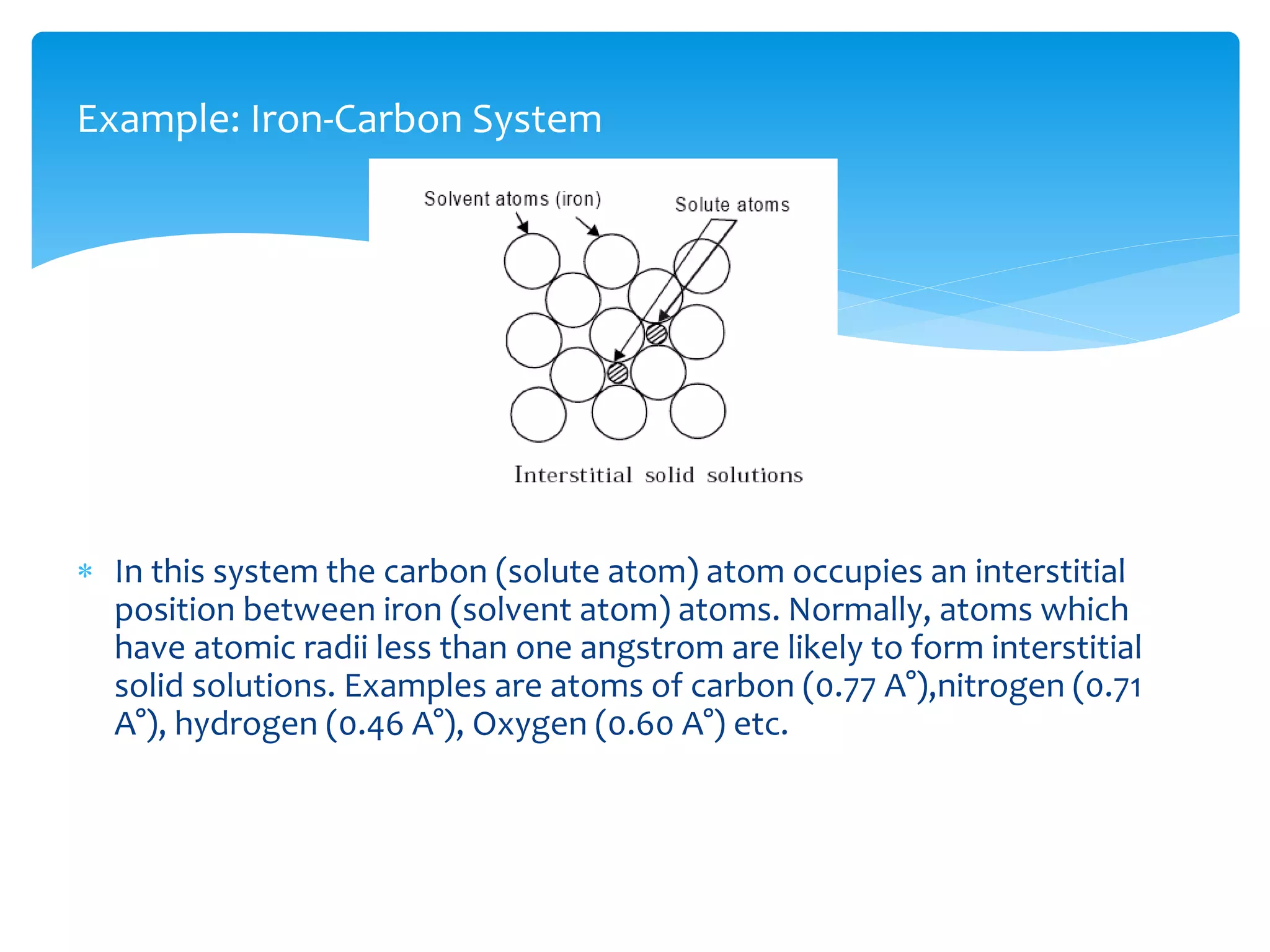
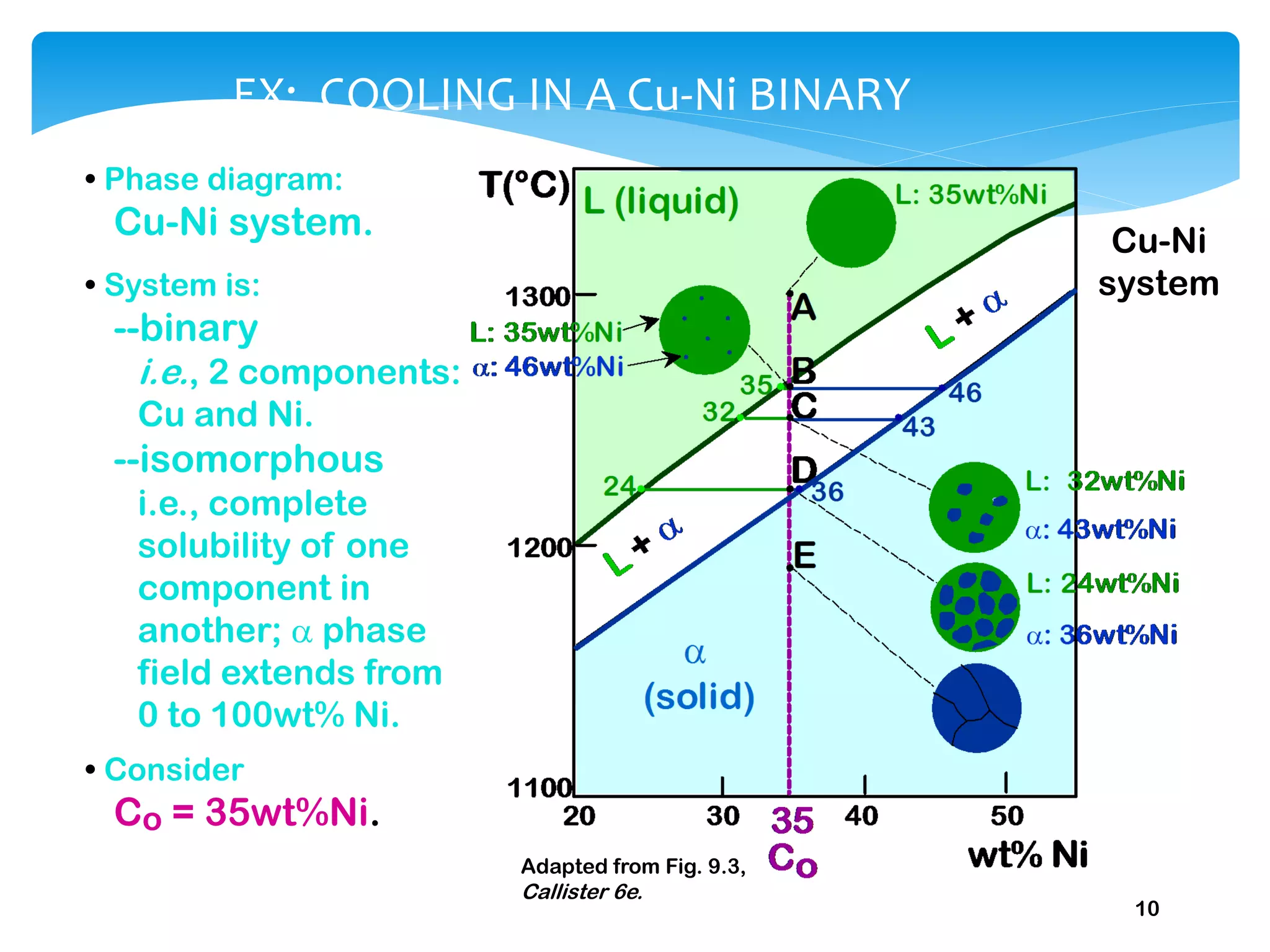
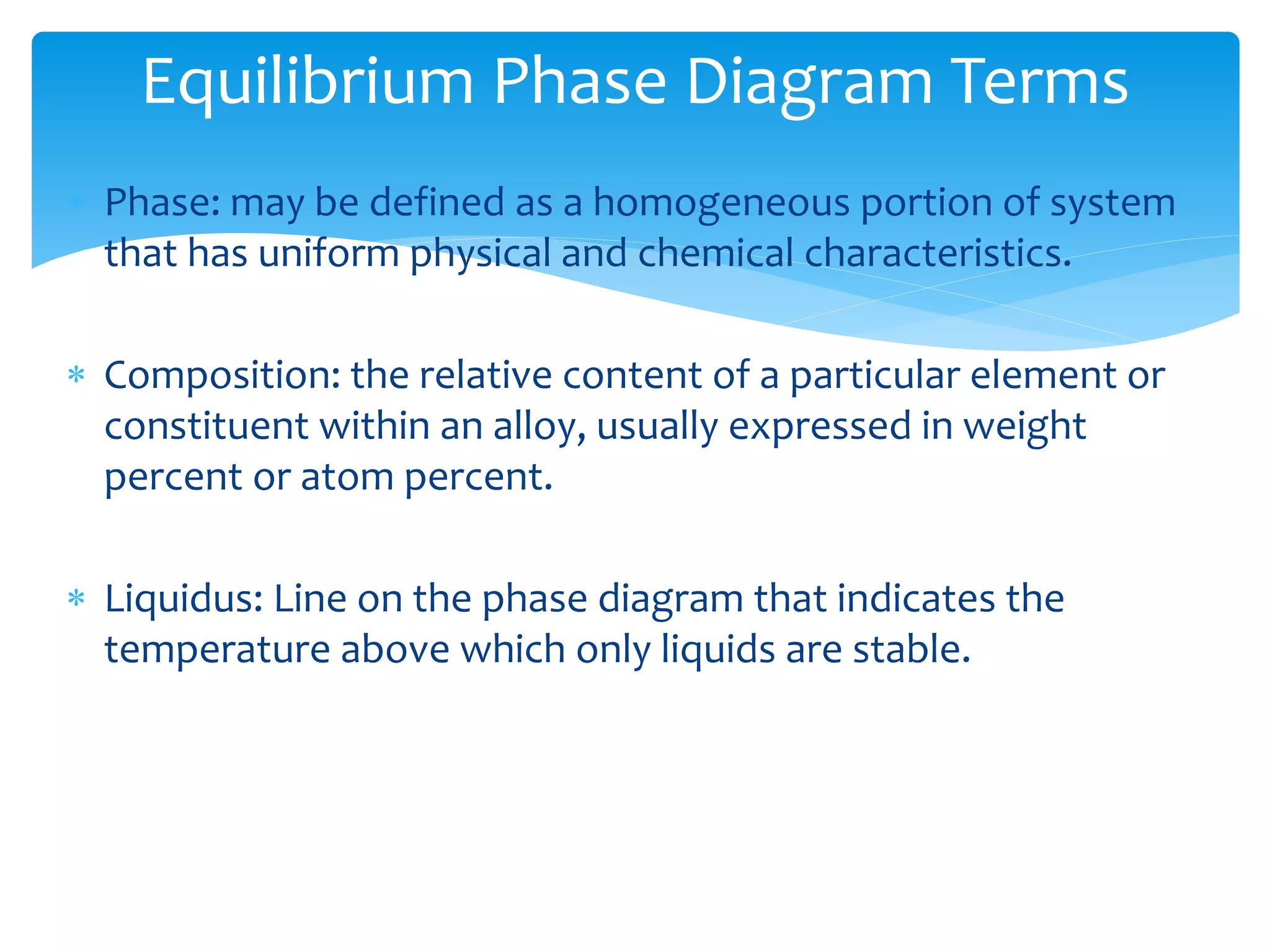
1) Elements are pure substances made of one type of atom, while compounds are made of two or more elements chemically bonded together. Mixtures are combinations of substances mixed but not chemically bonded.
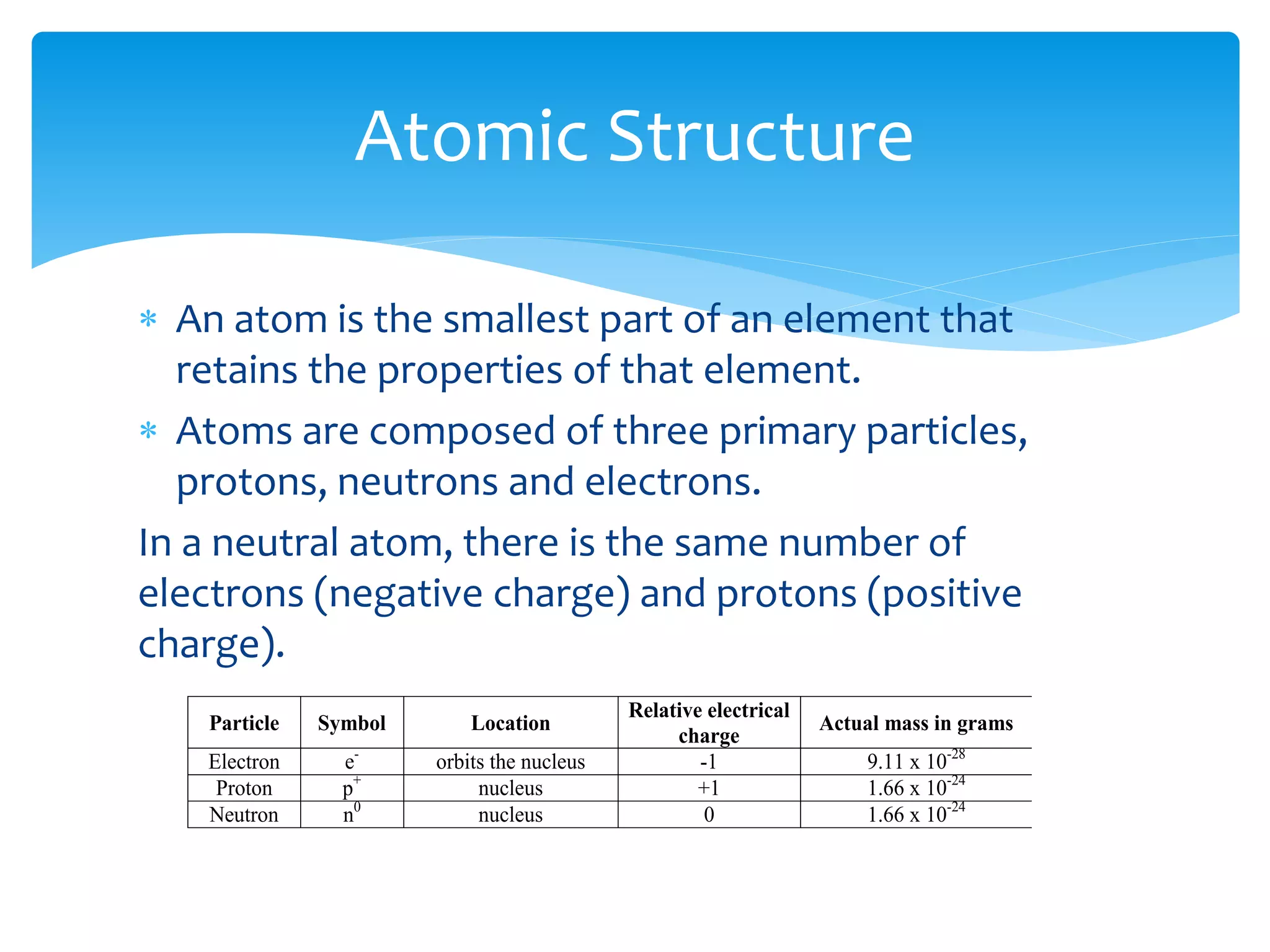
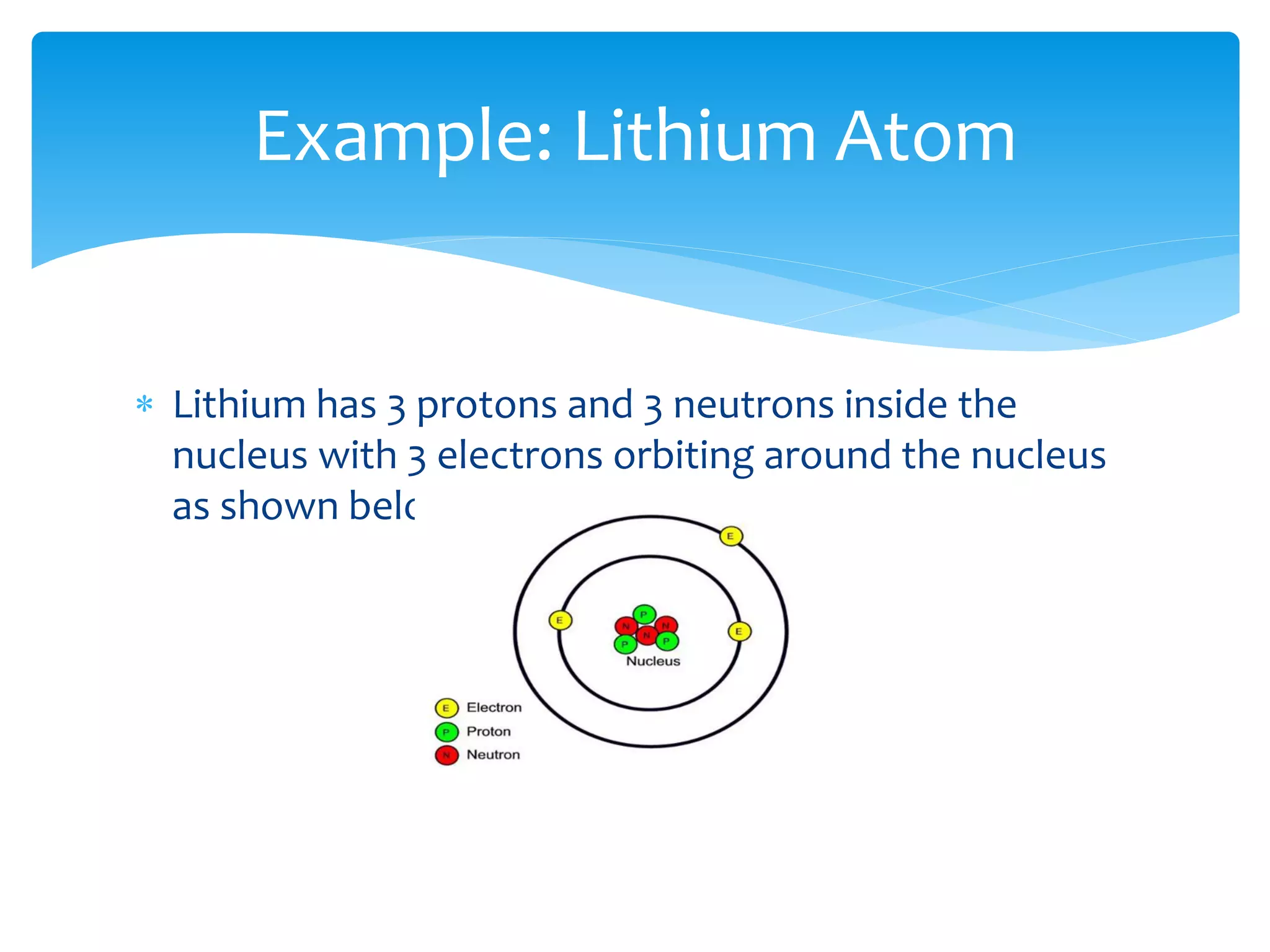
2) Atoms are made up of protons, neutrons, and electrons. The atomic structure of an element determines its properties.
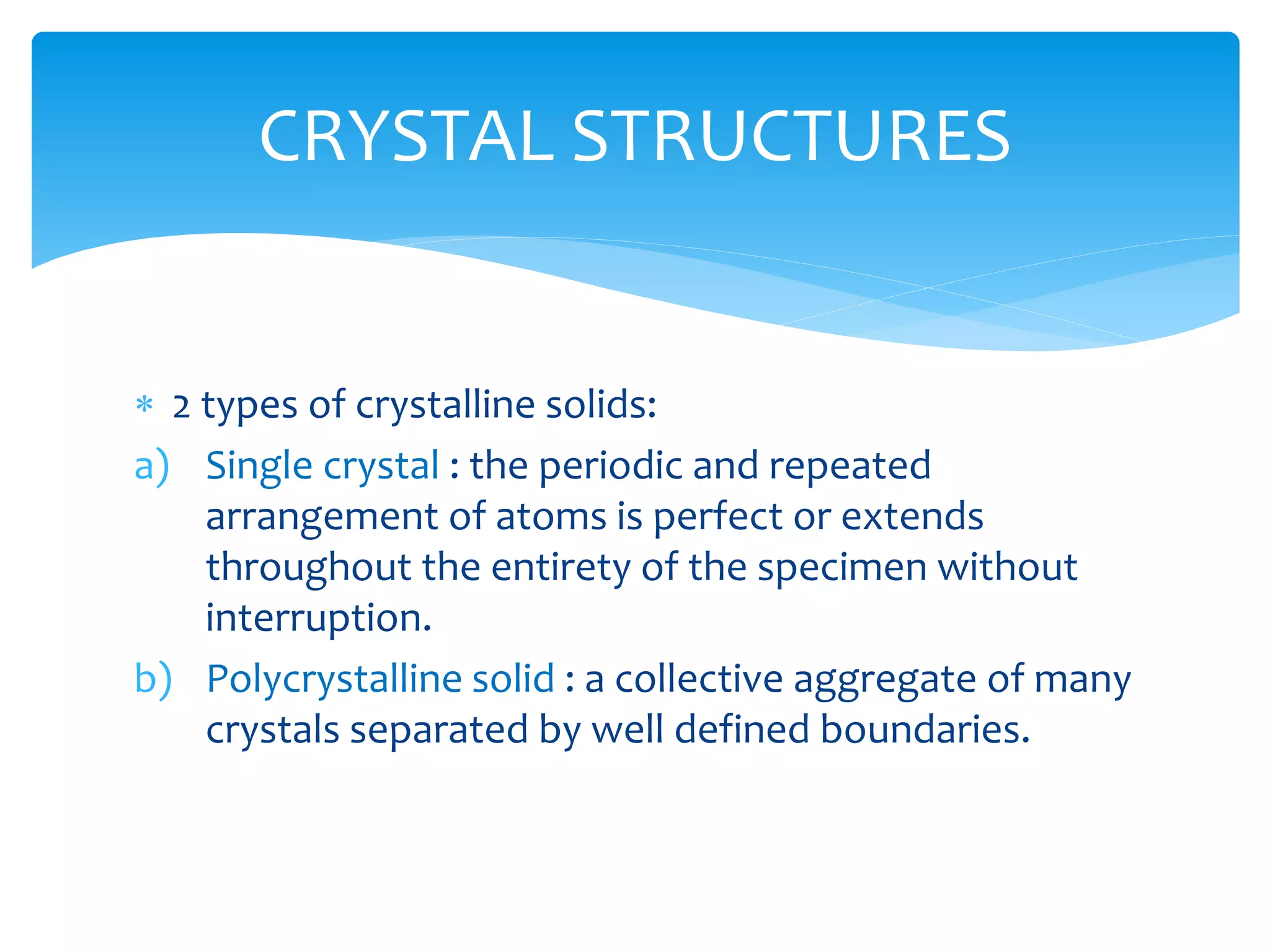
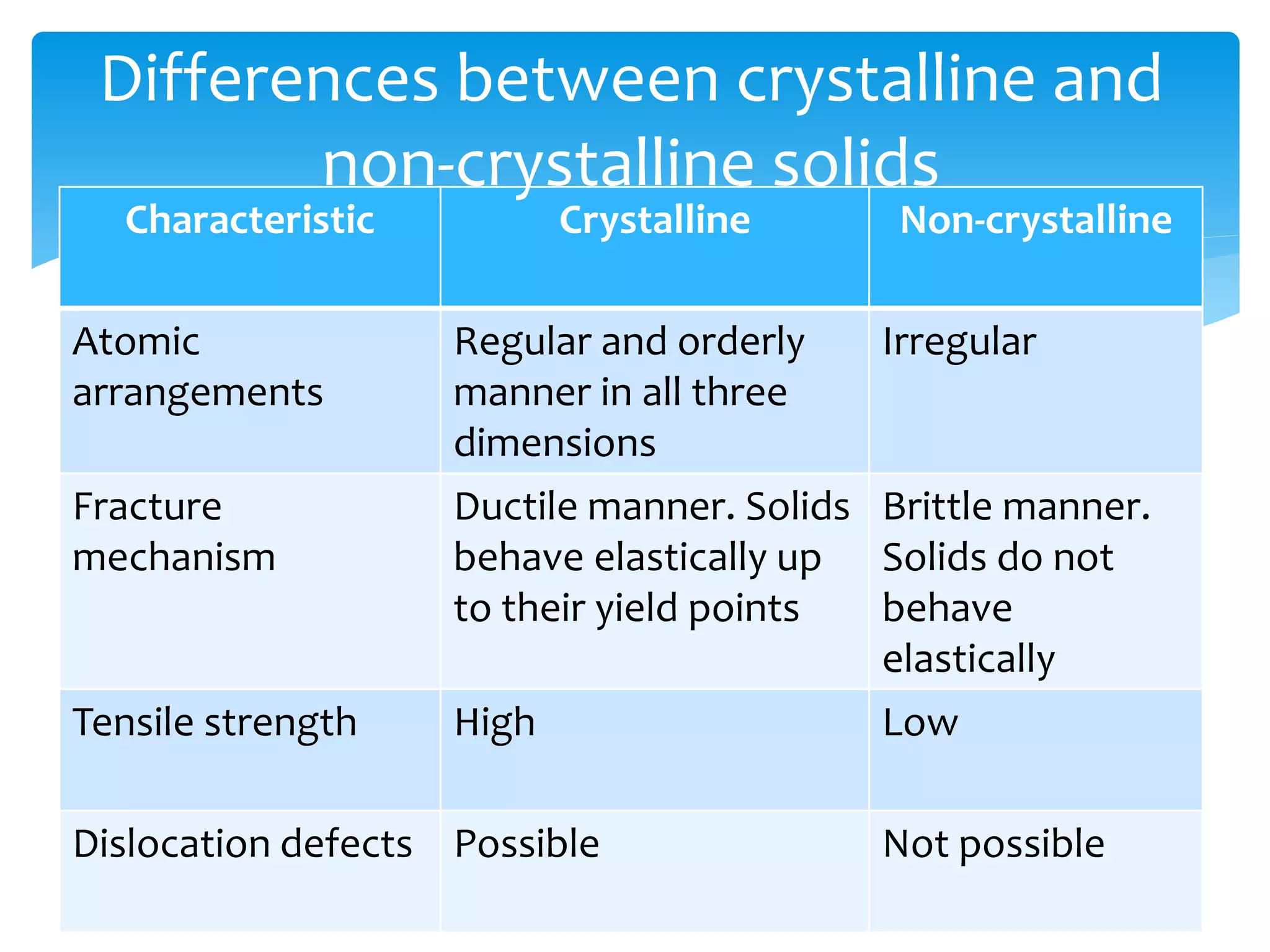
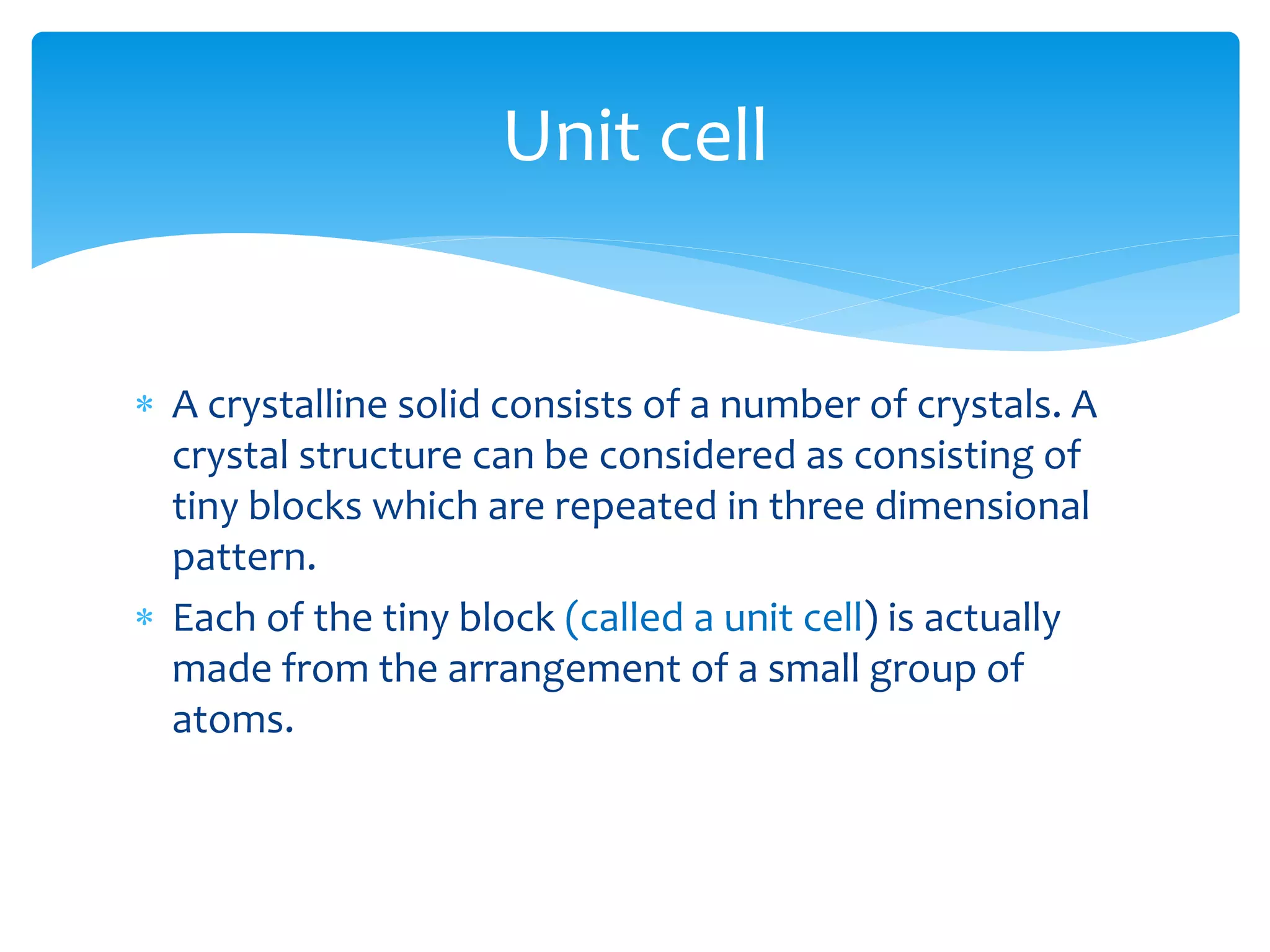
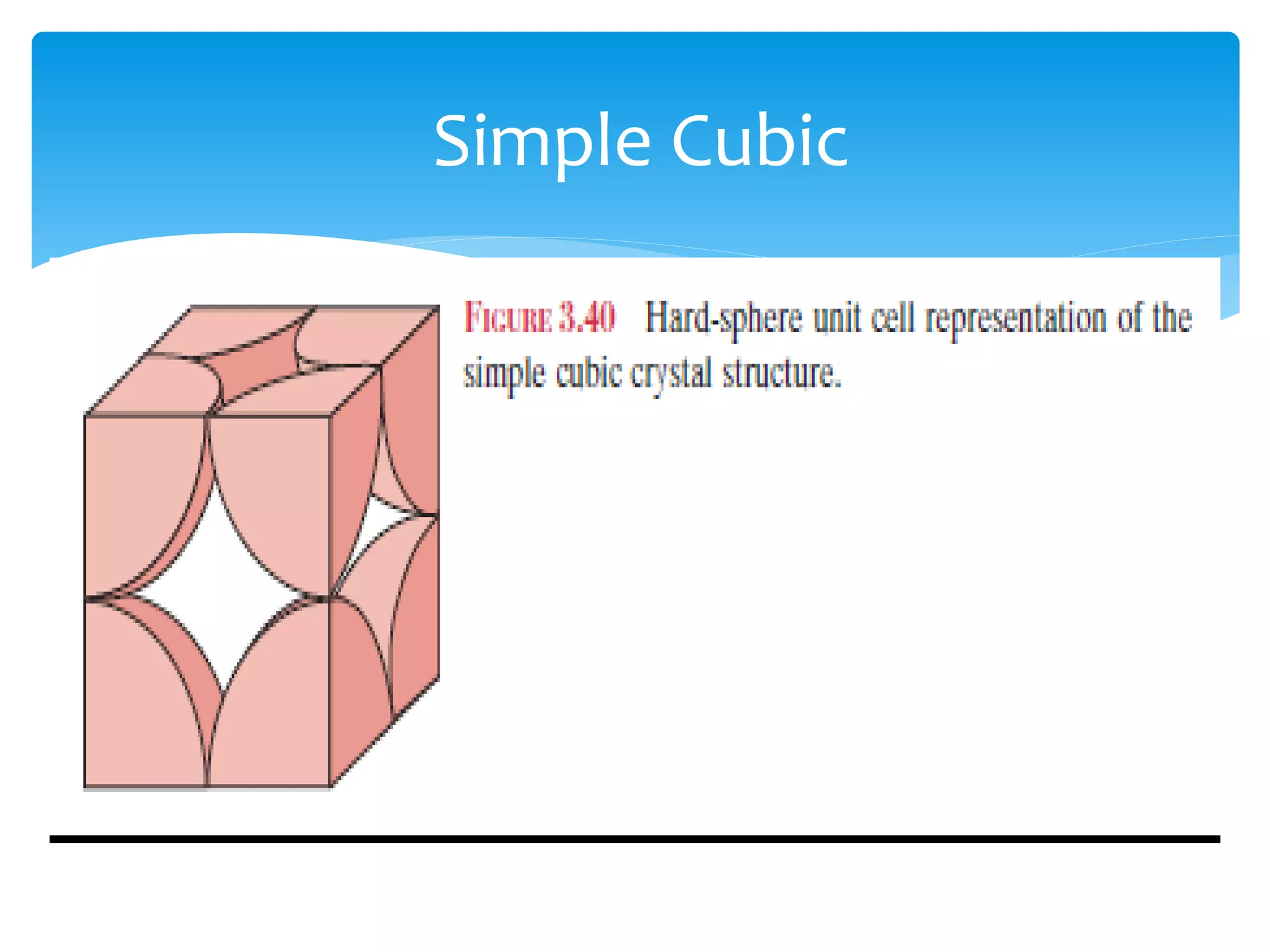
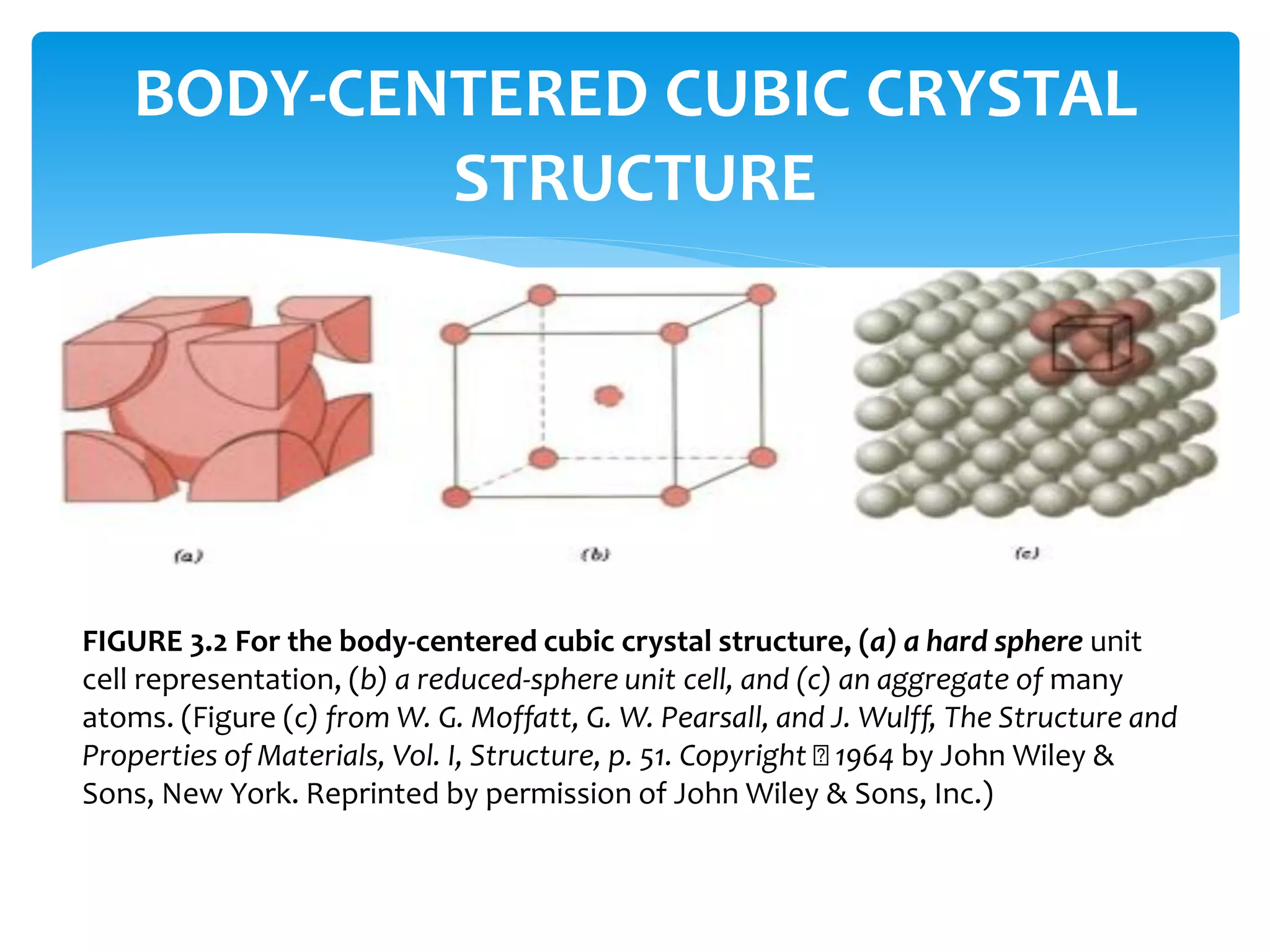
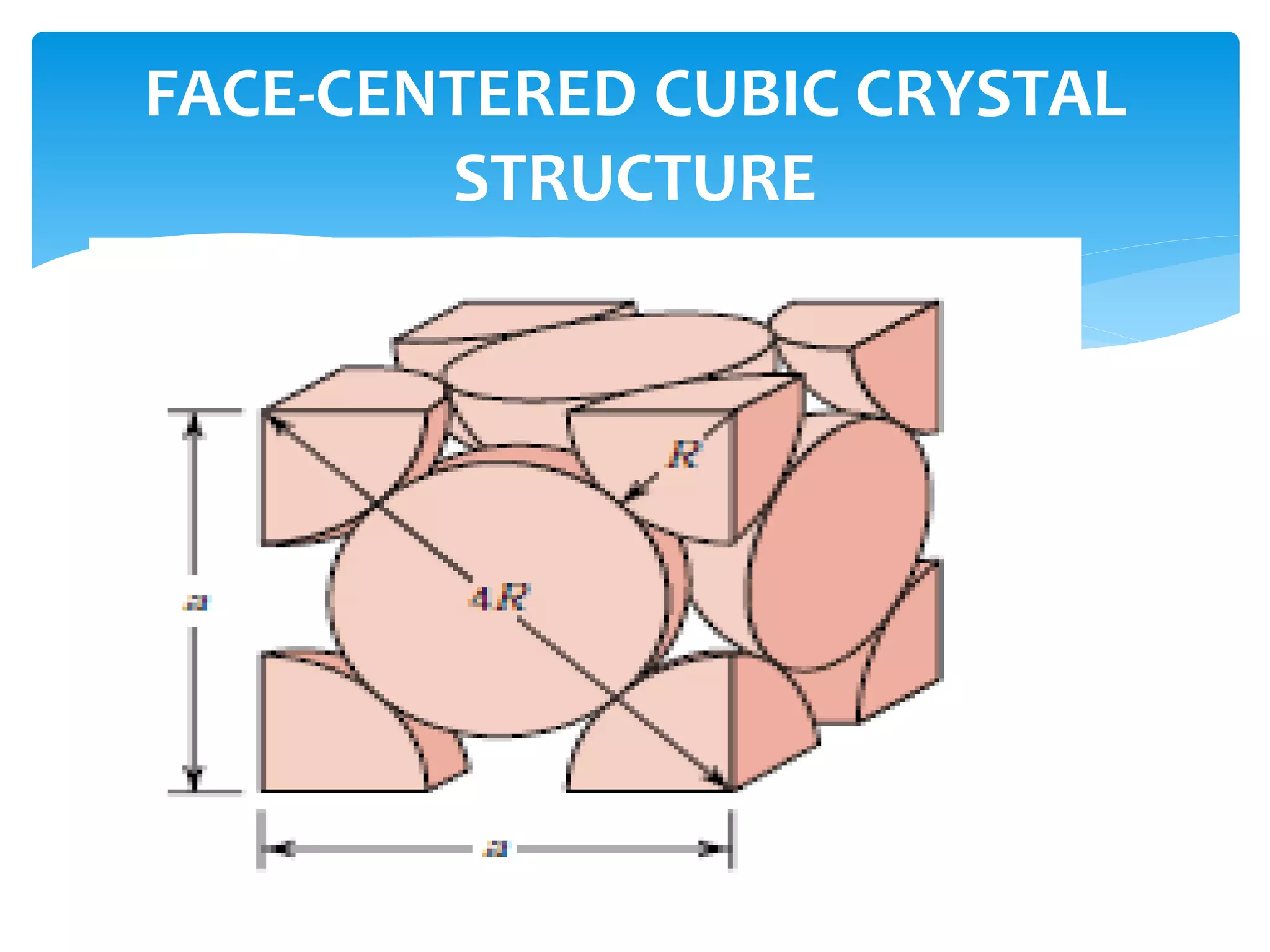
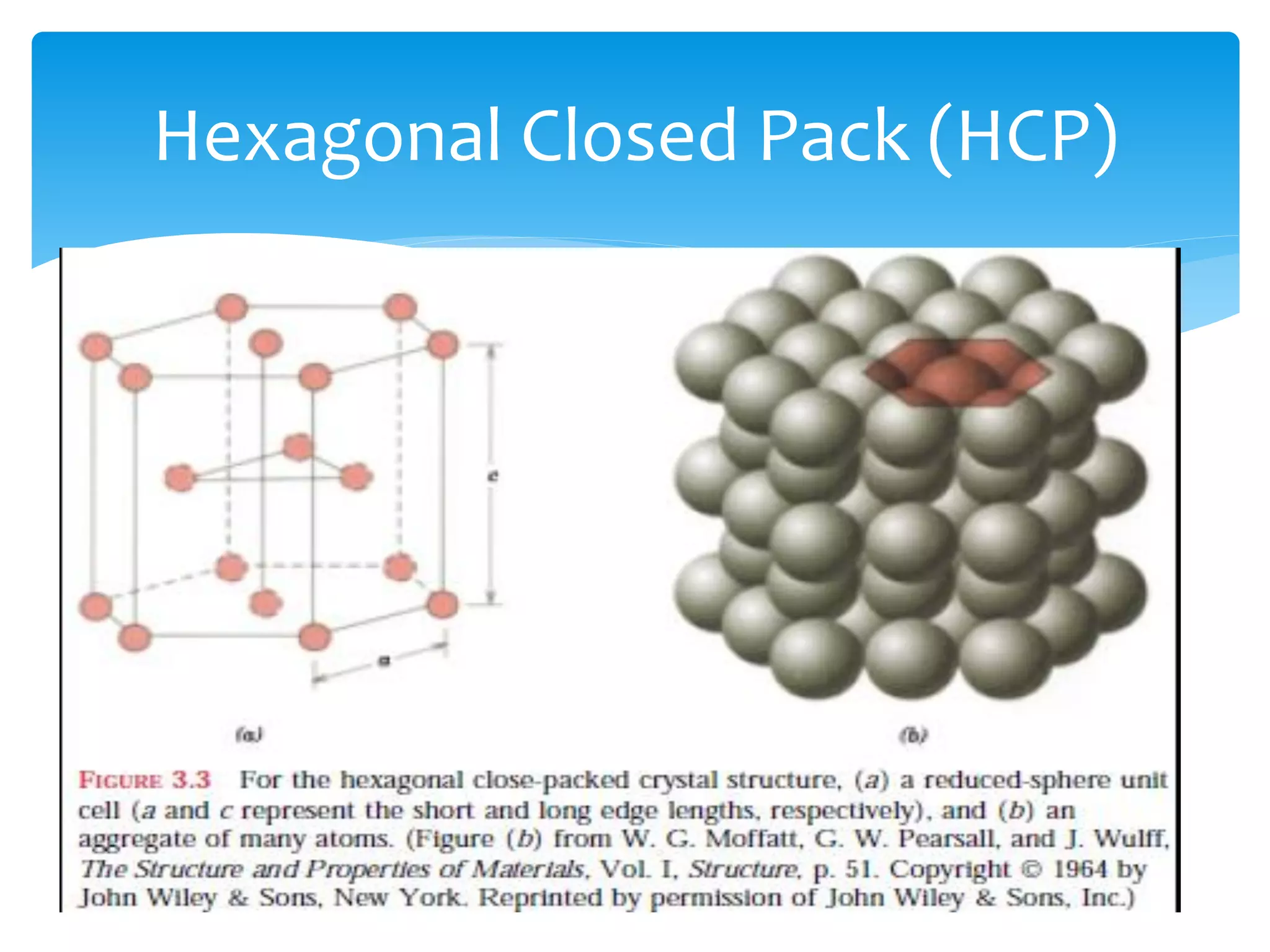
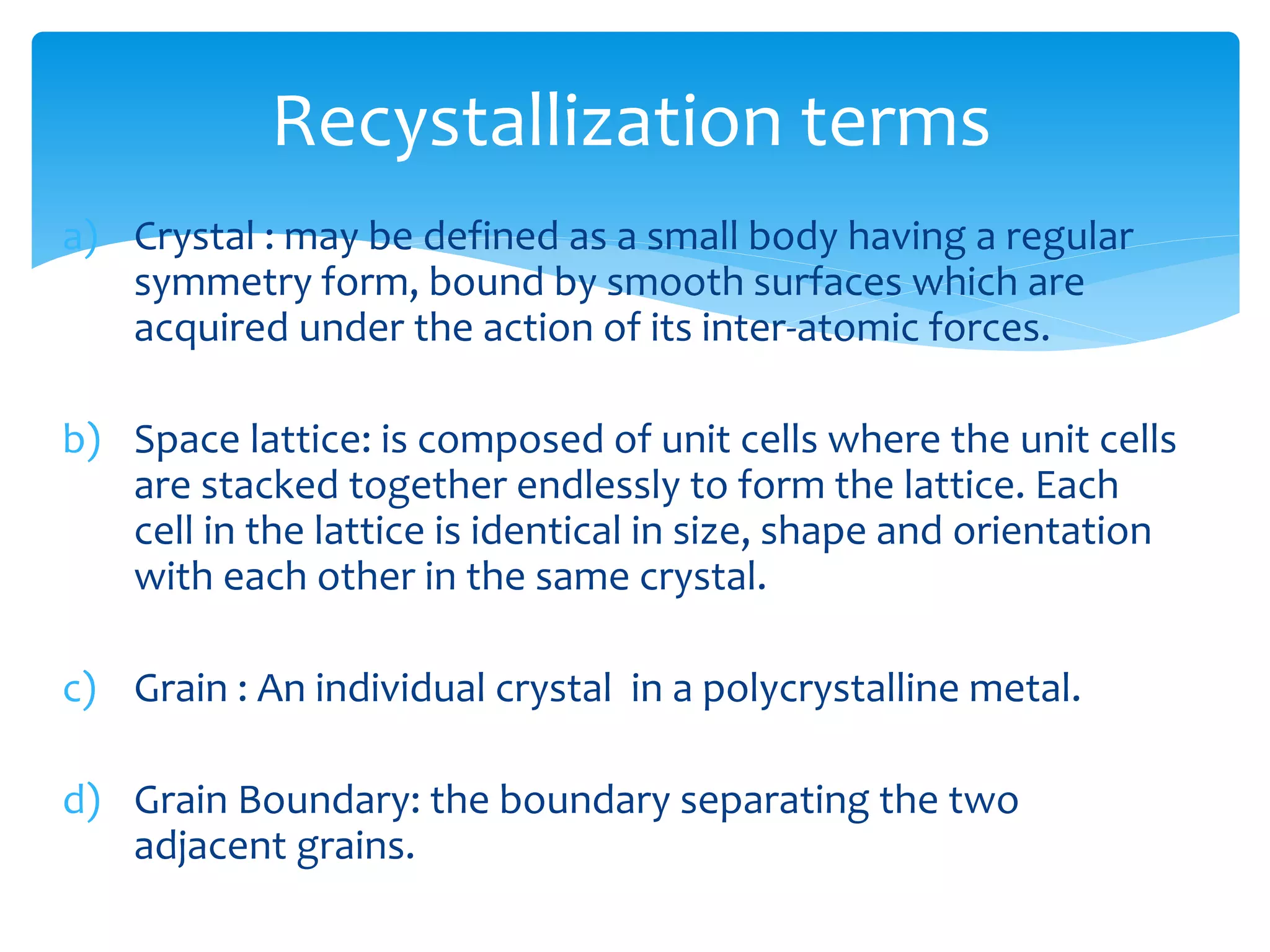
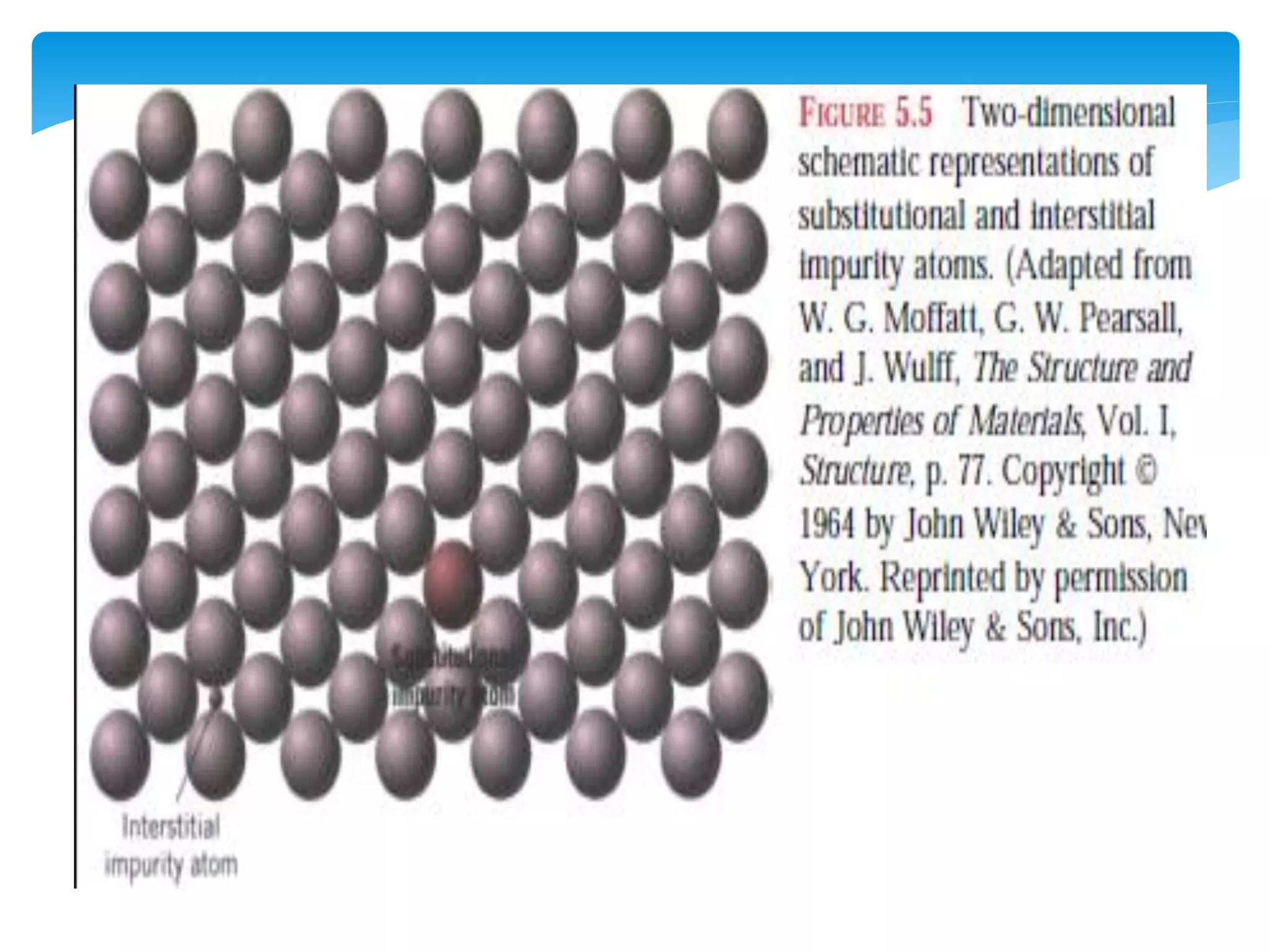
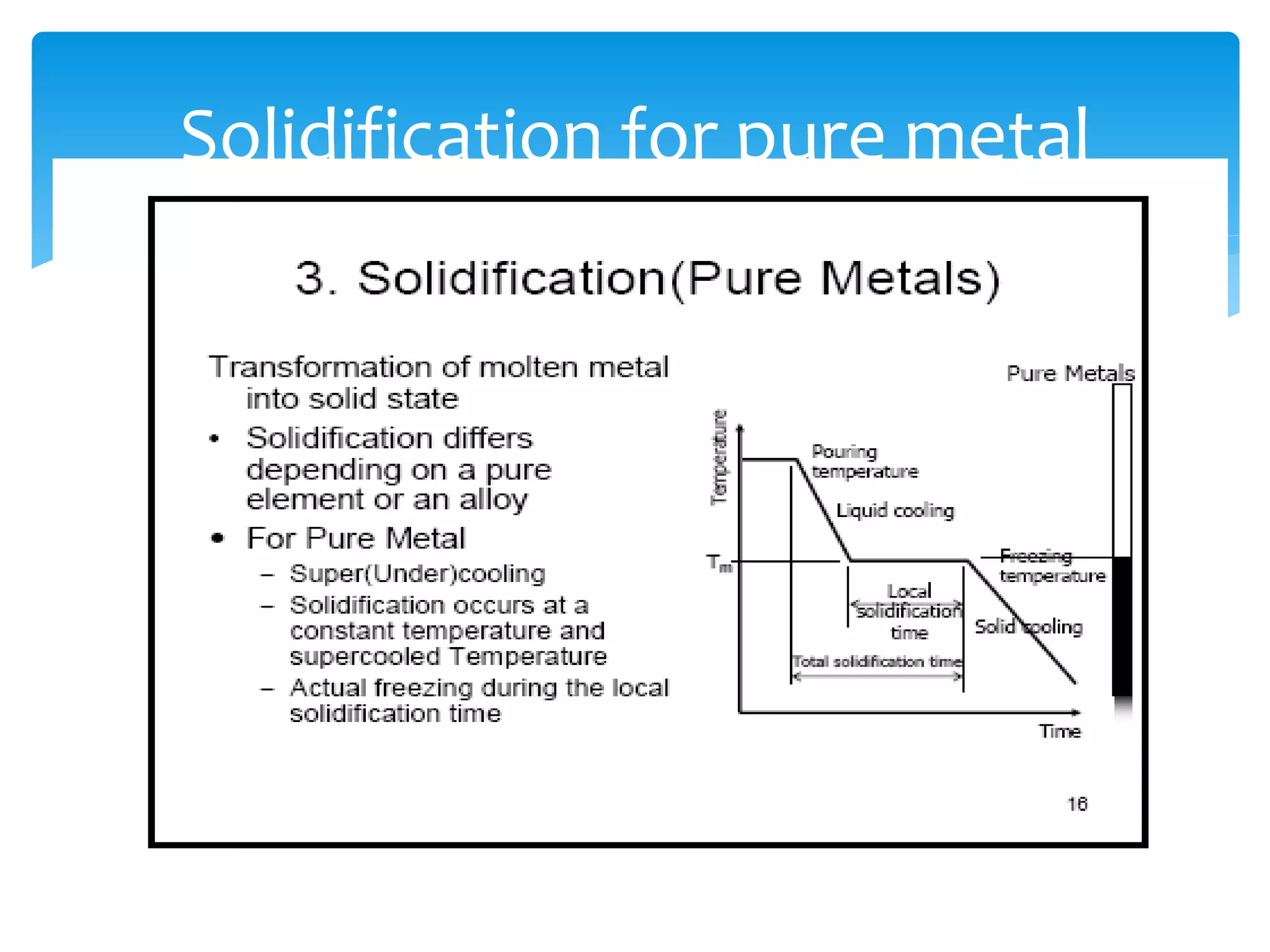
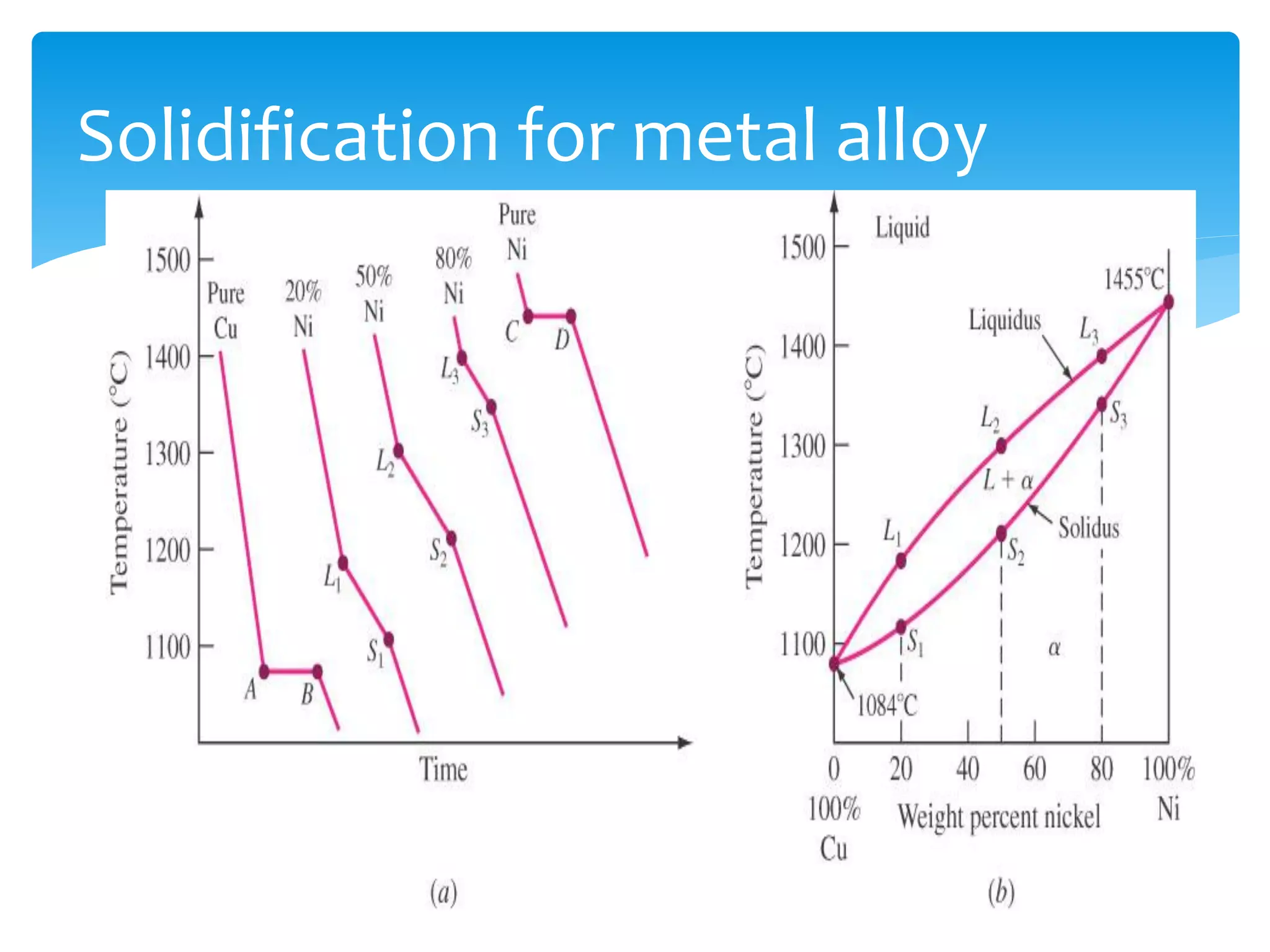
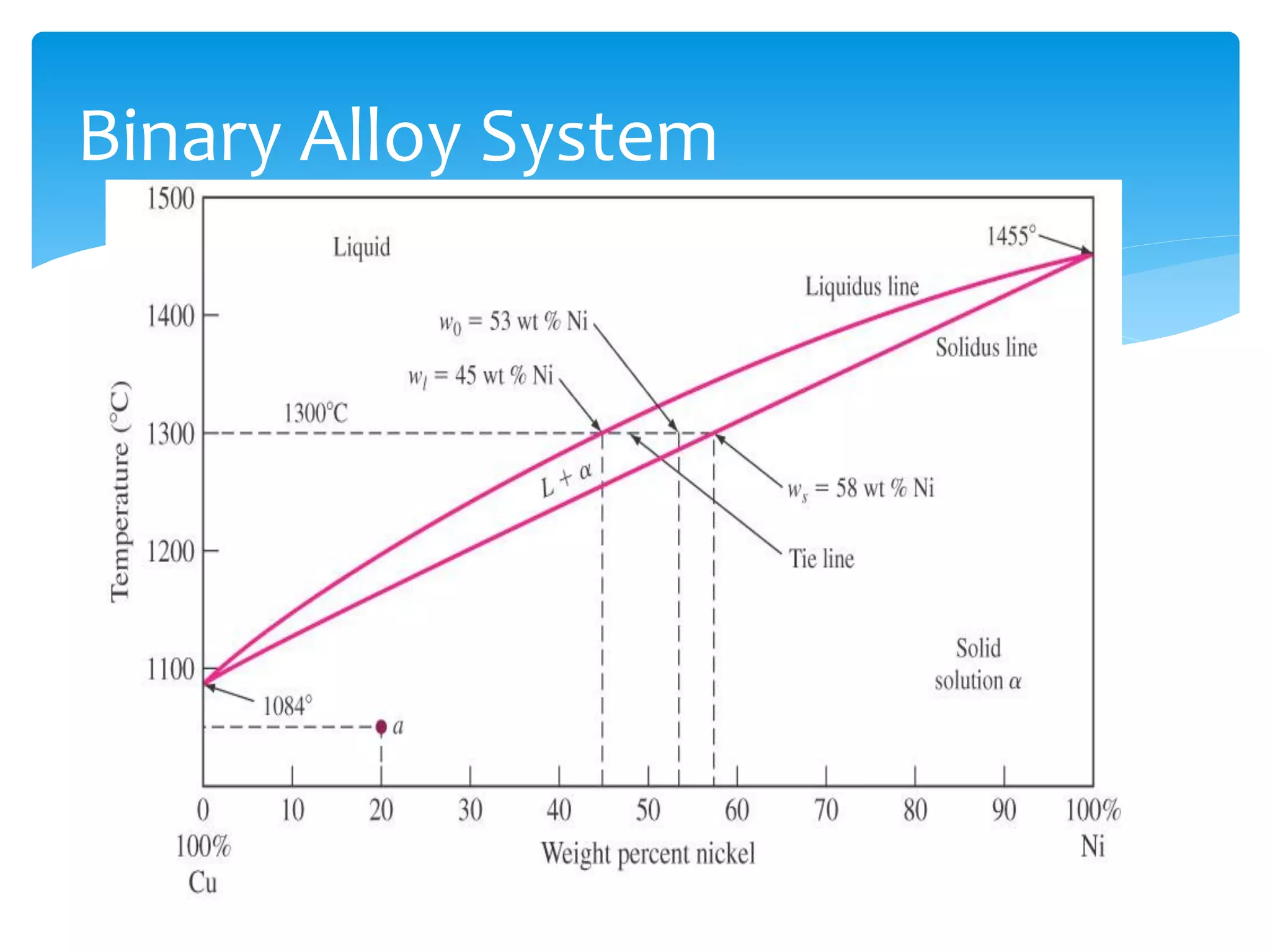
3) Materials can have crystalline or non-crystalline structures. Crystalline structures are regular arrangements of atoms, while non-crystalline structures are irregular. The type of bonding between atoms also influences properties.