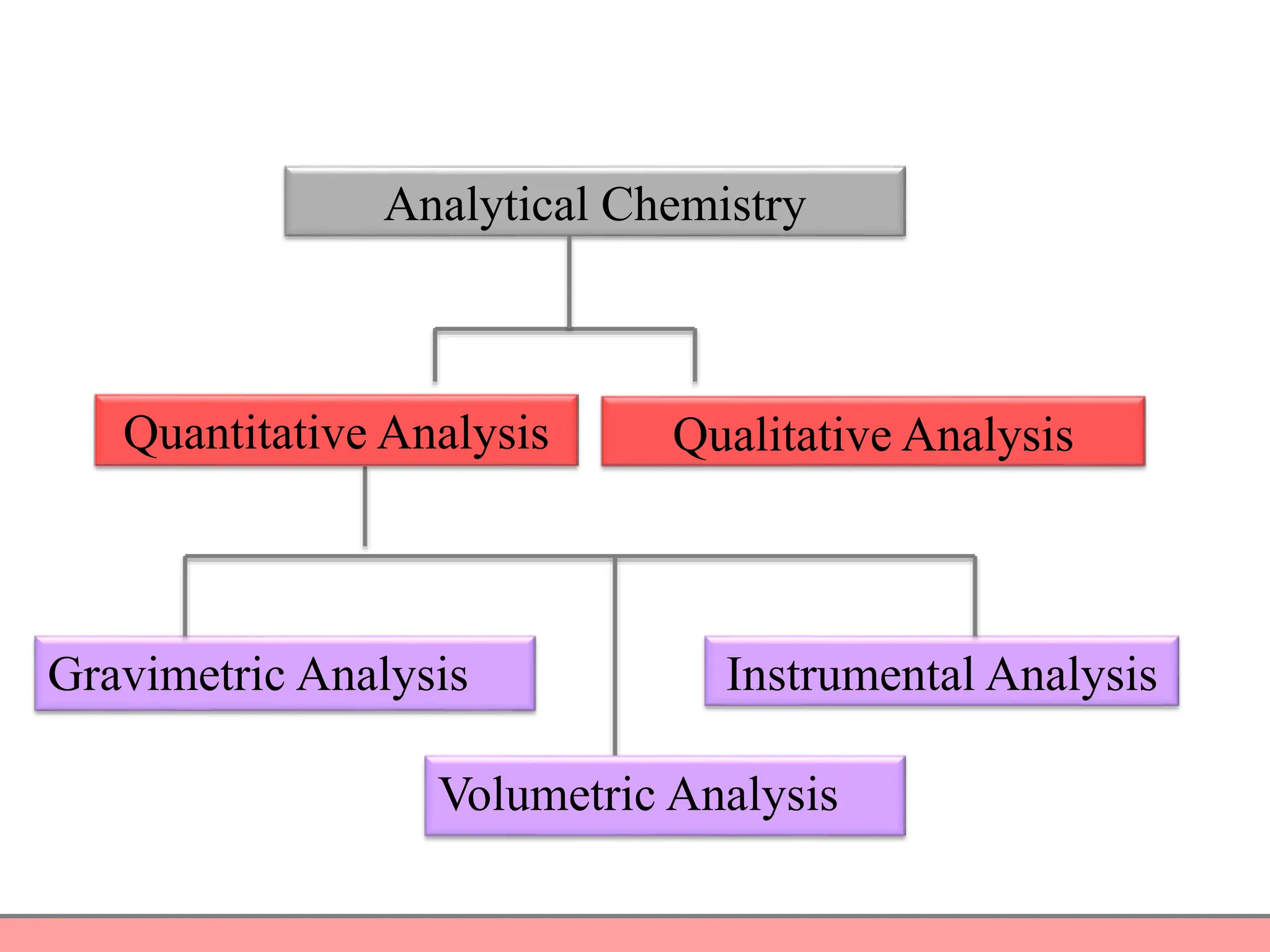
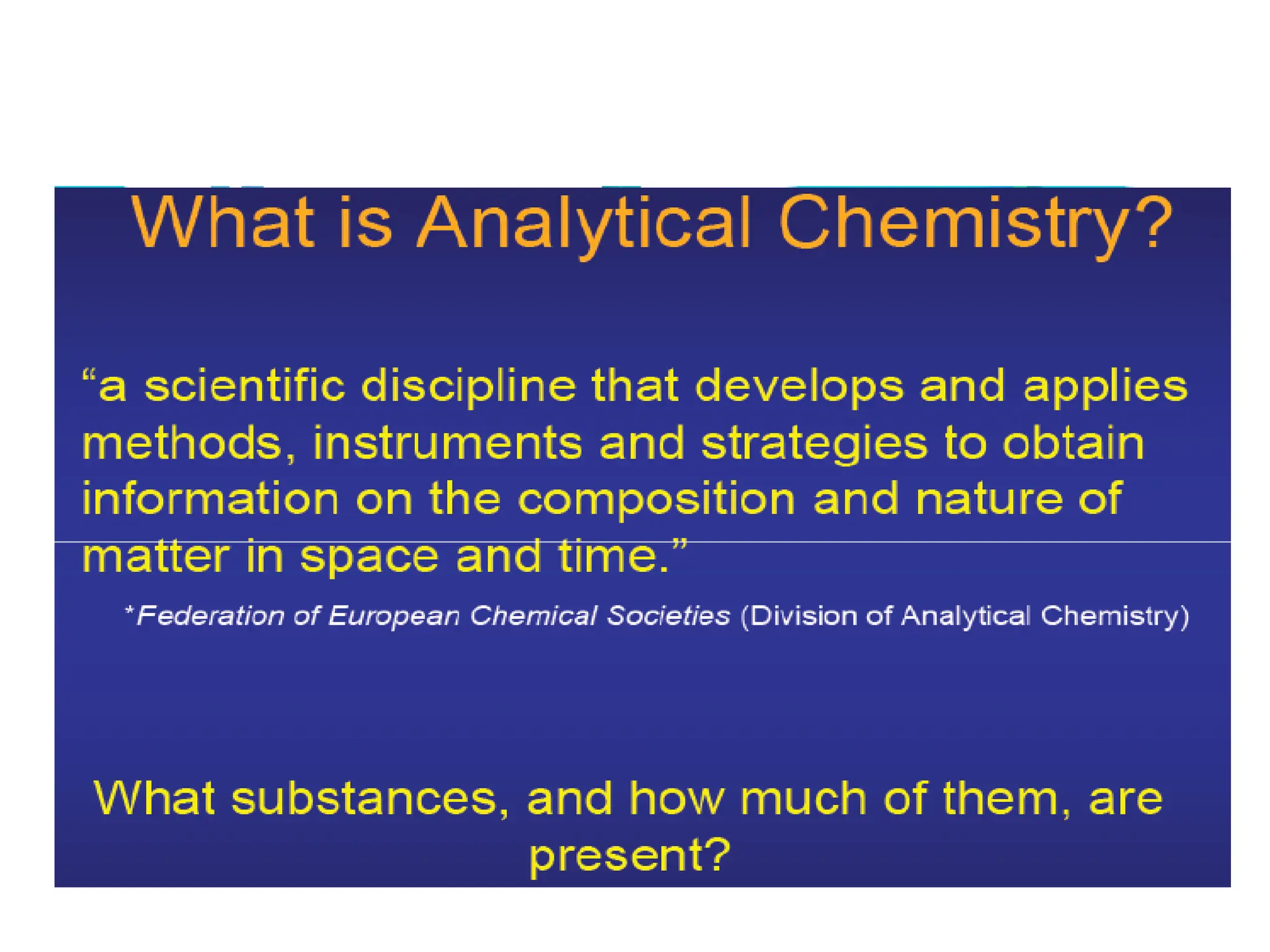
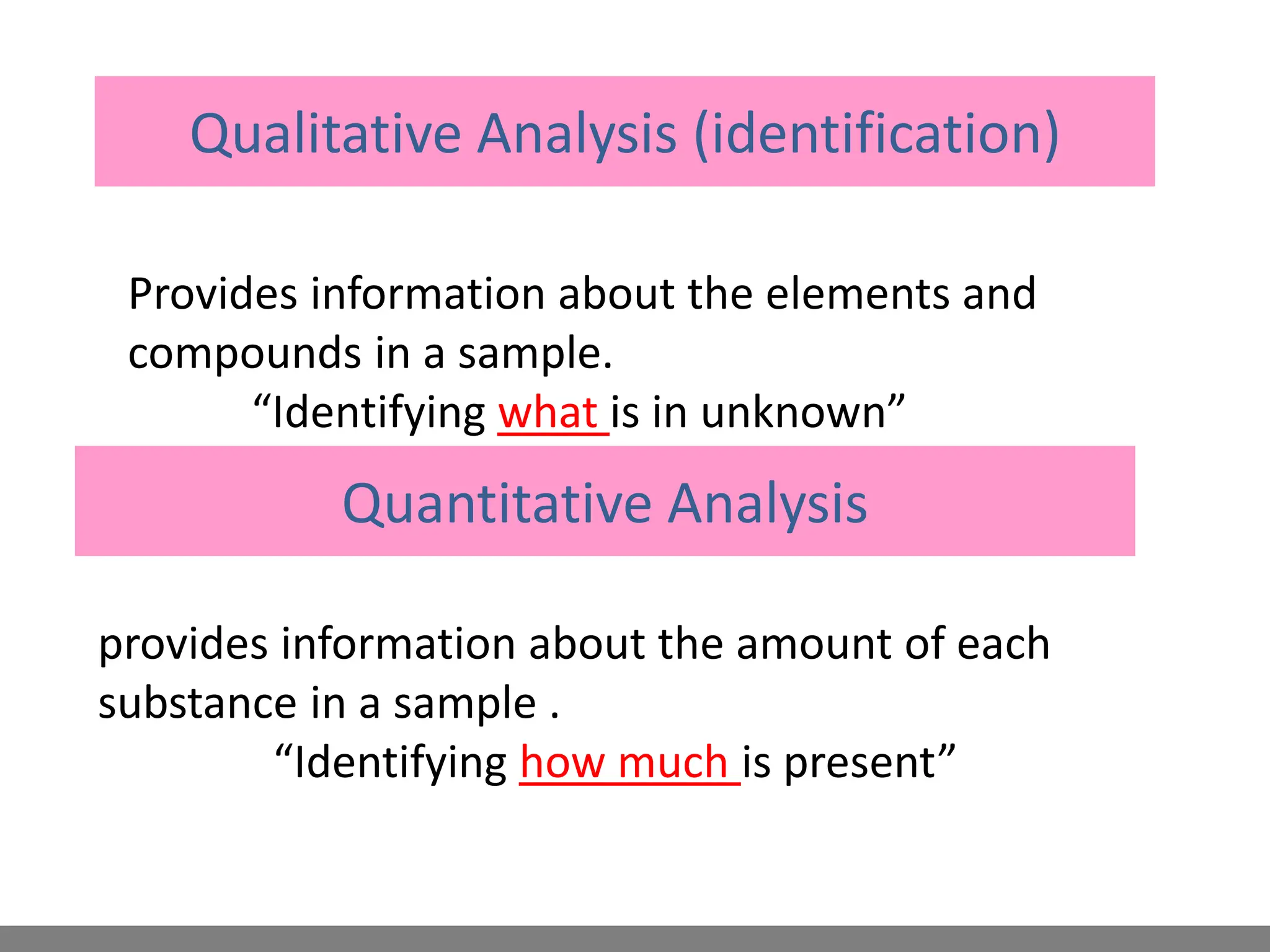
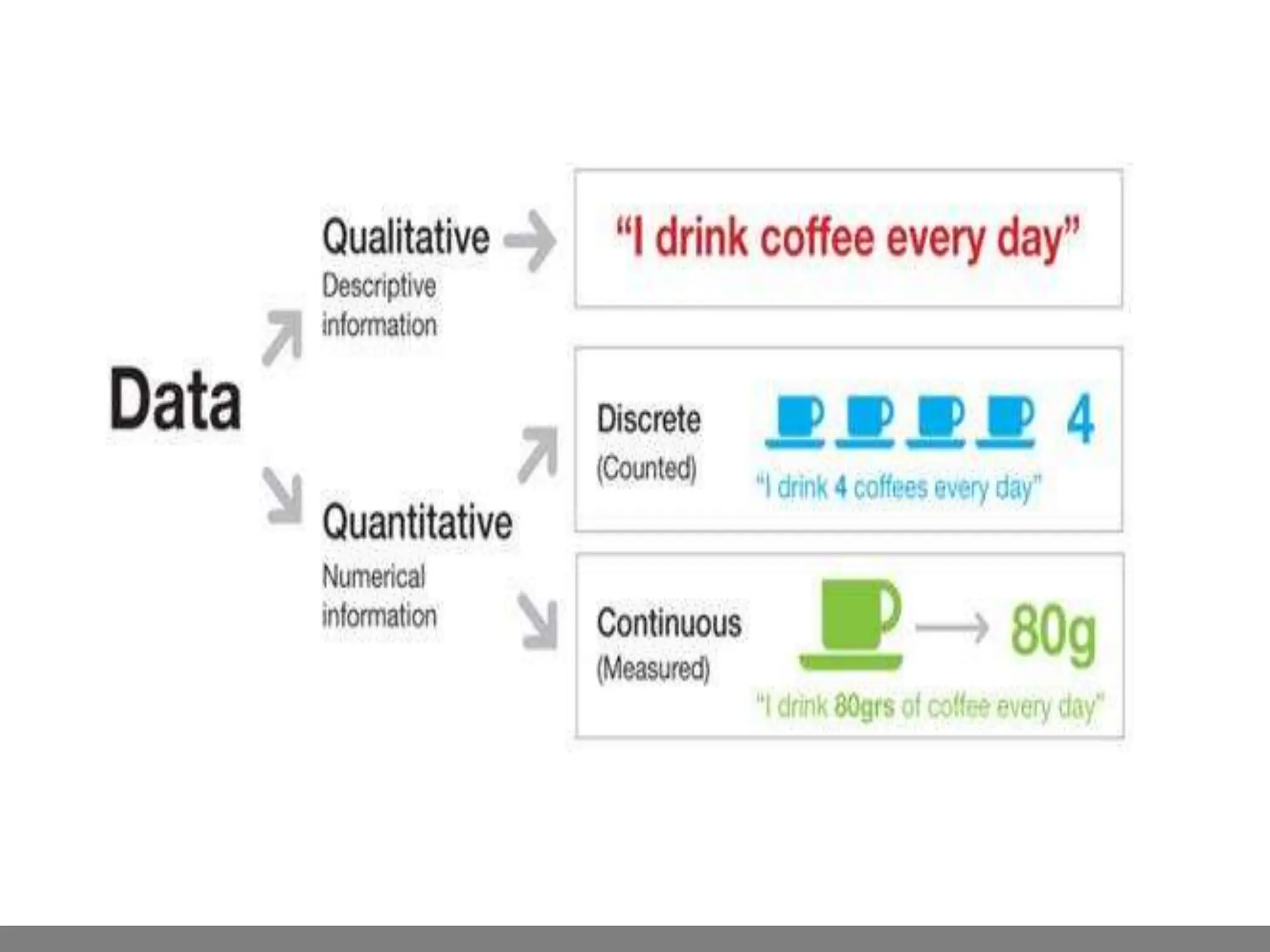
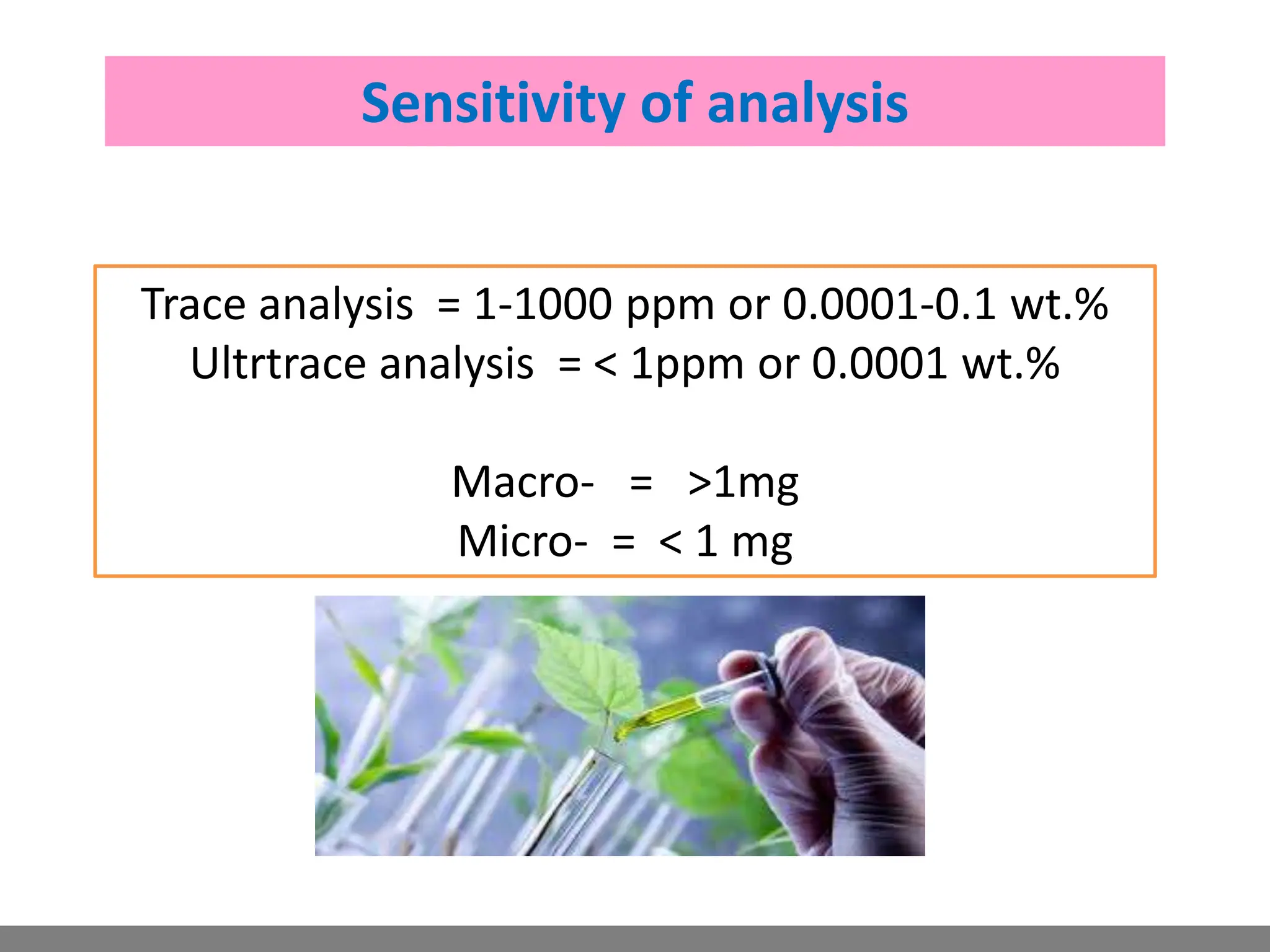
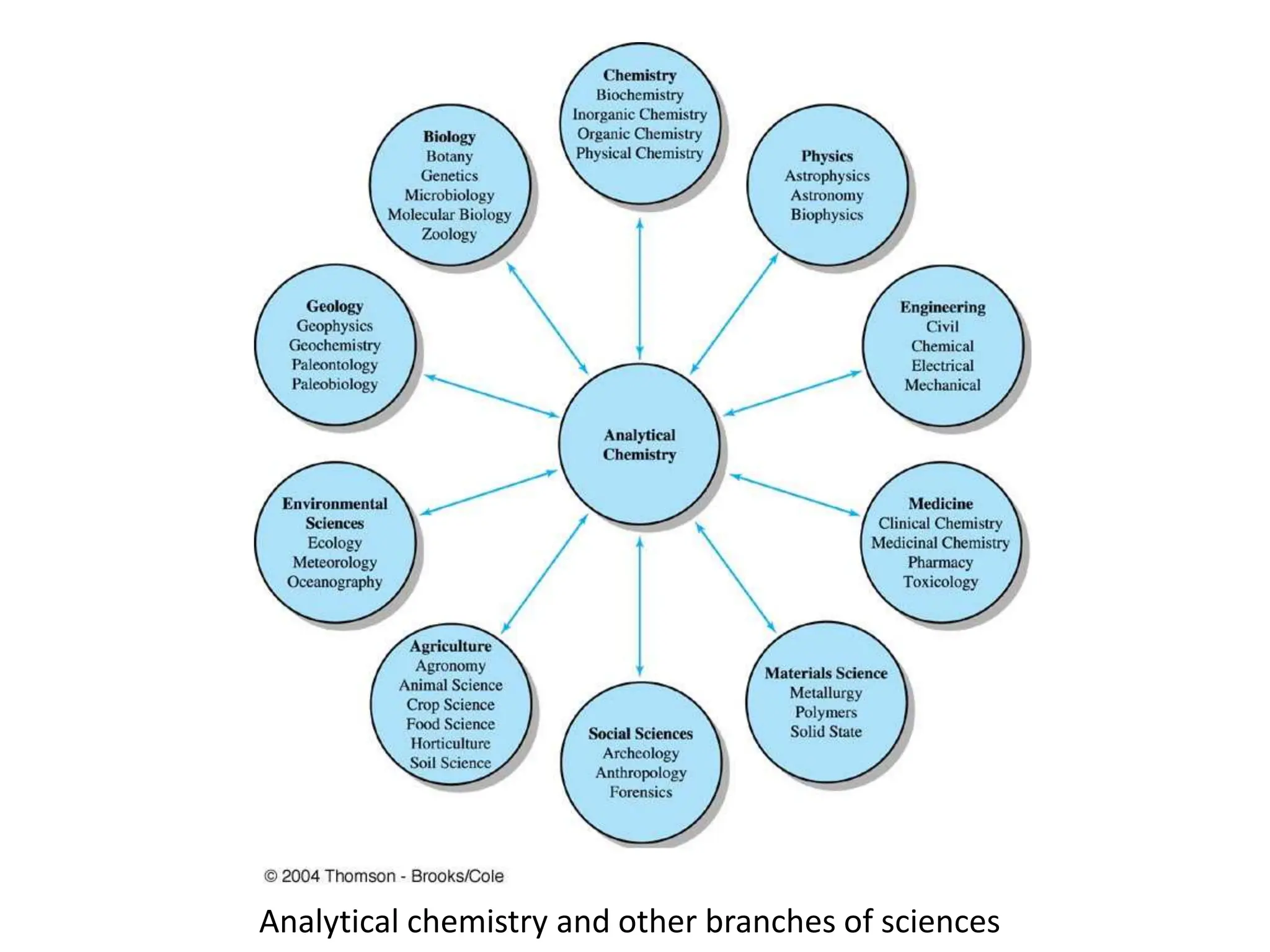
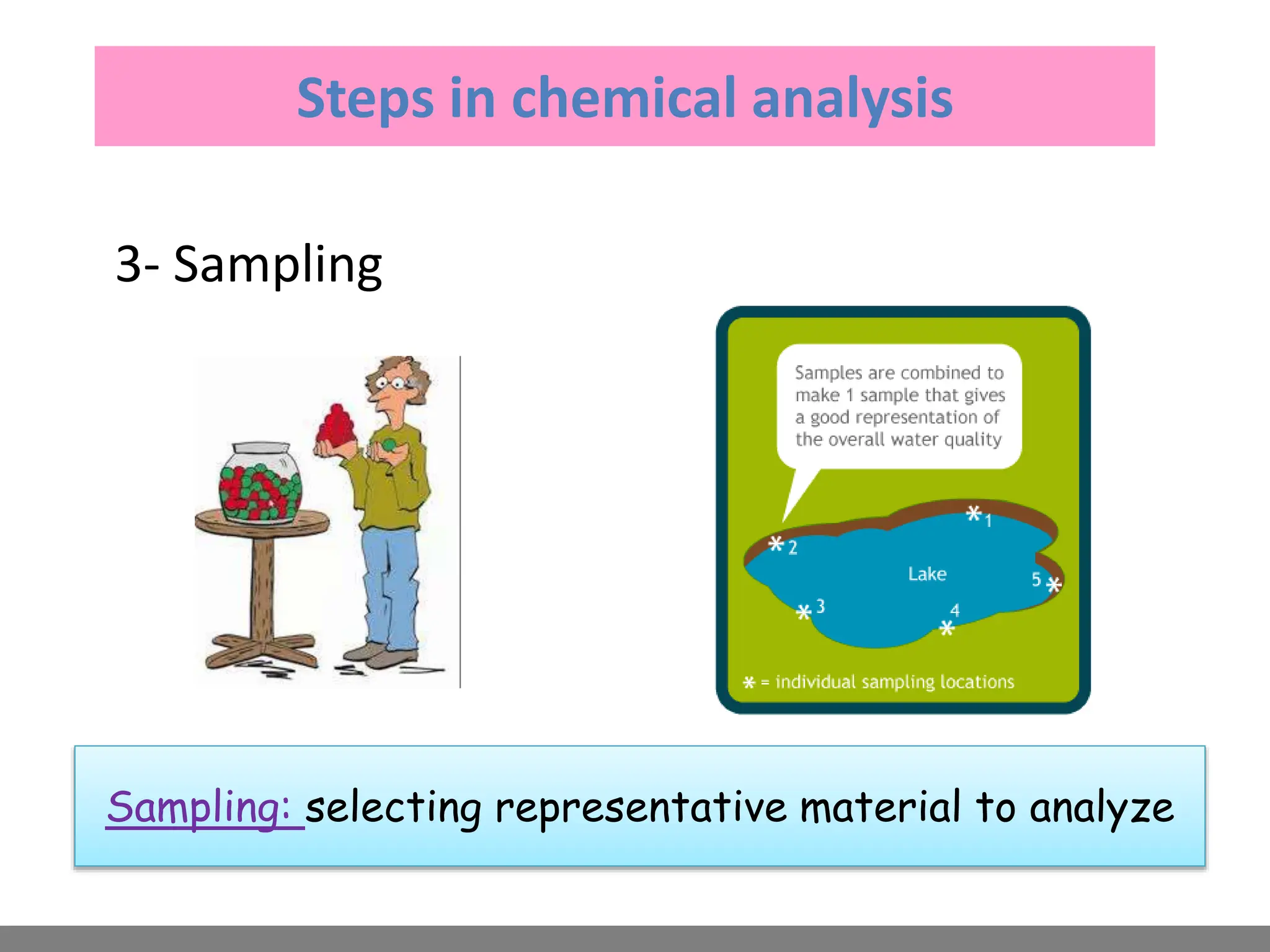
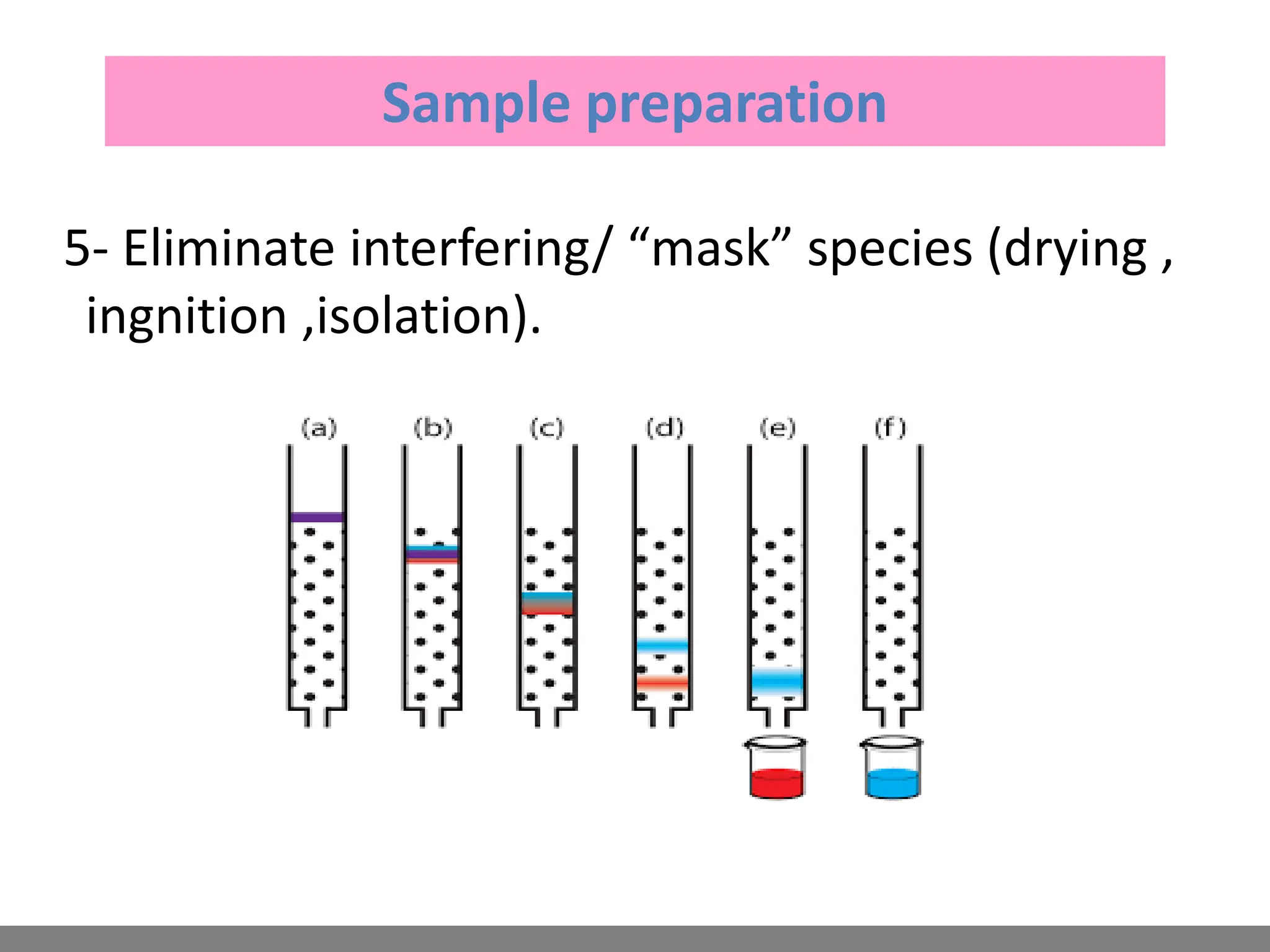
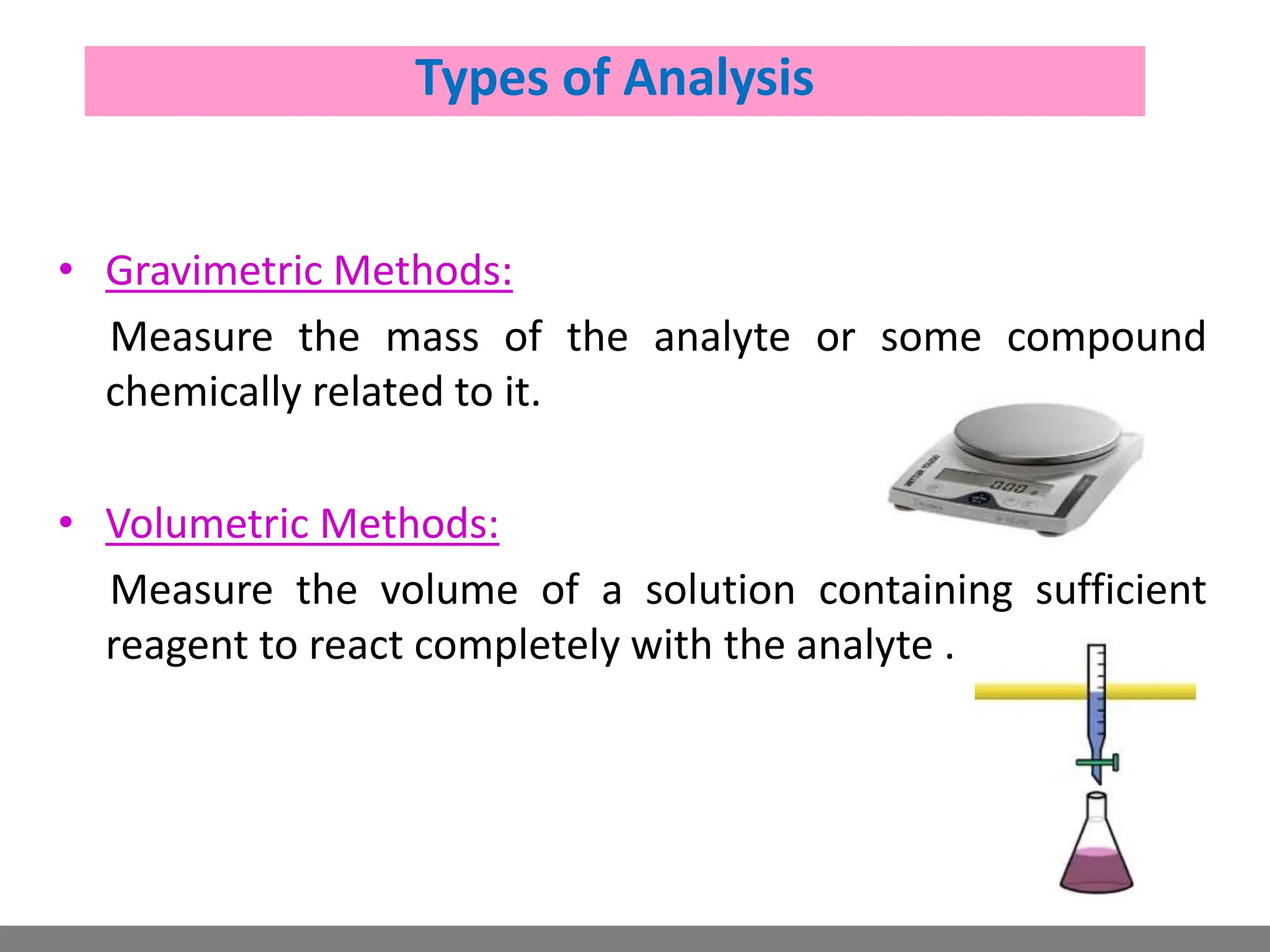
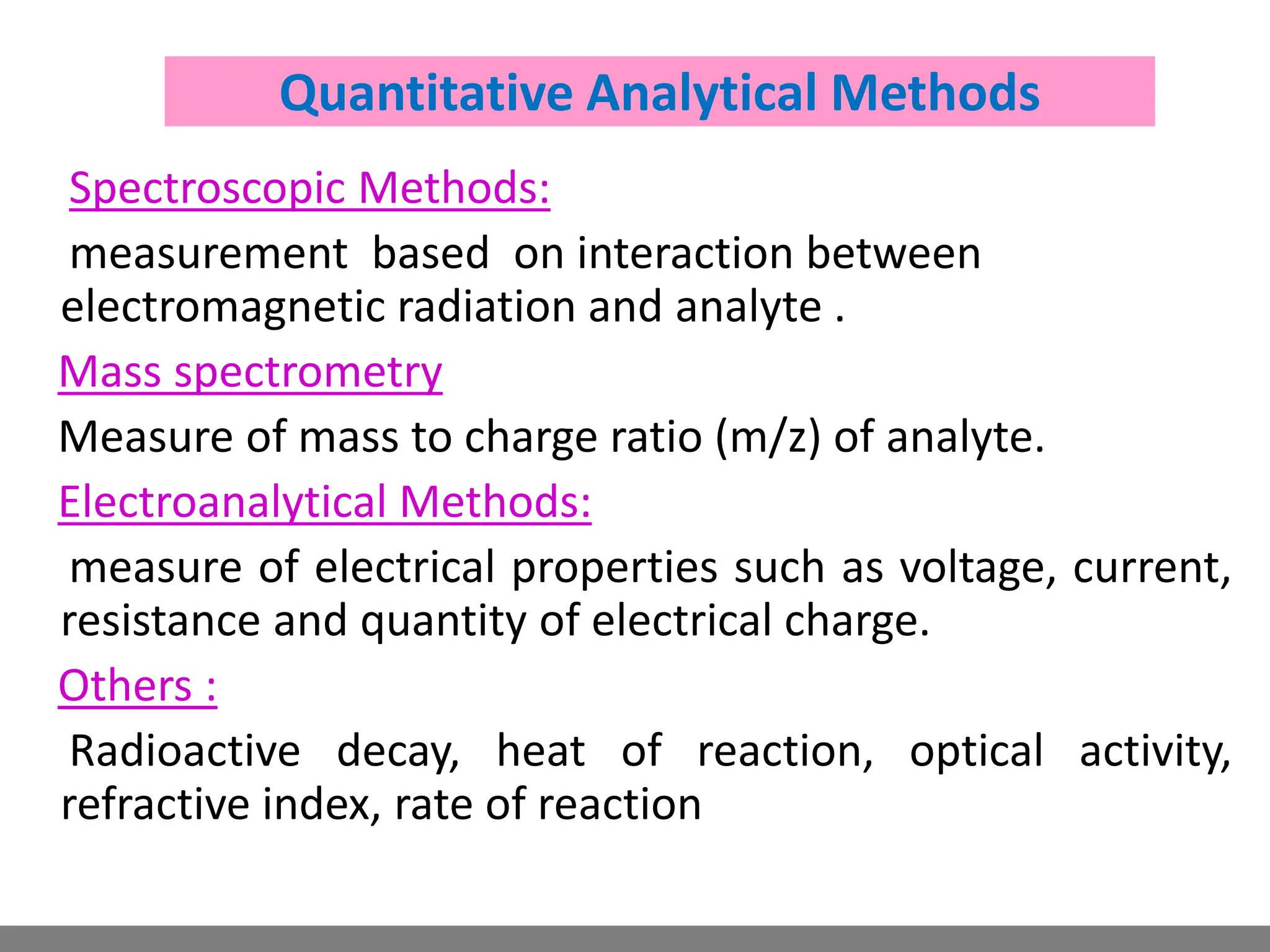
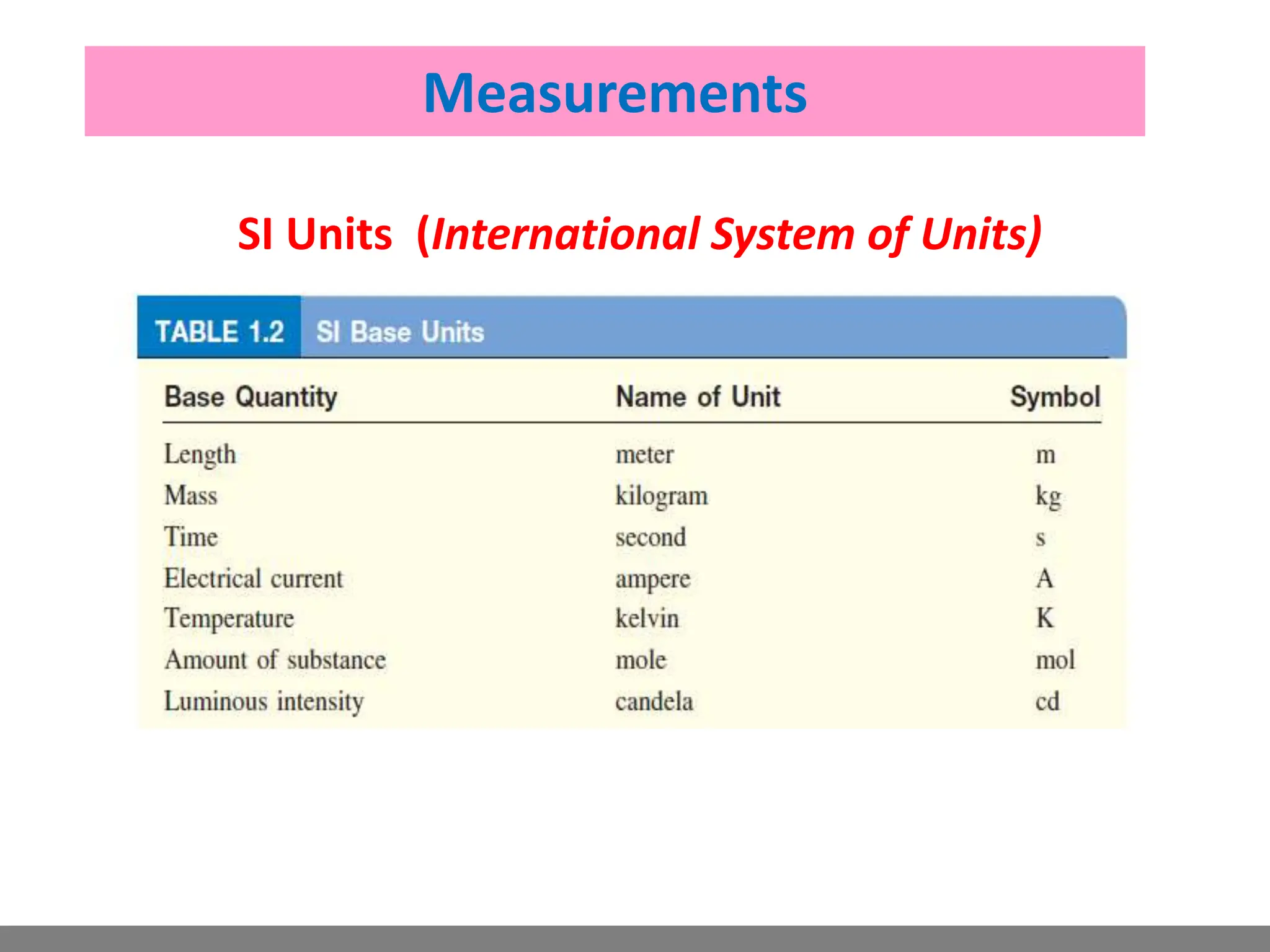
Analytical chemistry involves qualitative and quantitative analysis. Qualitative analysis identifies elements and compounds in a sample, while quantitative analysis determines the amount present. Analytical chemistry plays a vital role across many fields by measuring substances at trace levels down to parts per million. It uses a variety of methods like gravimetric, volumetric, spectroscopic, electroanalytical and others to prepare samples and conduct analyses important for research, manufacturing, clinical labs, forensics and more.
![• The nature of analytical chemistry.
•]
• Qualitative and quantitative analysis.
• Sensitivity of analysis.
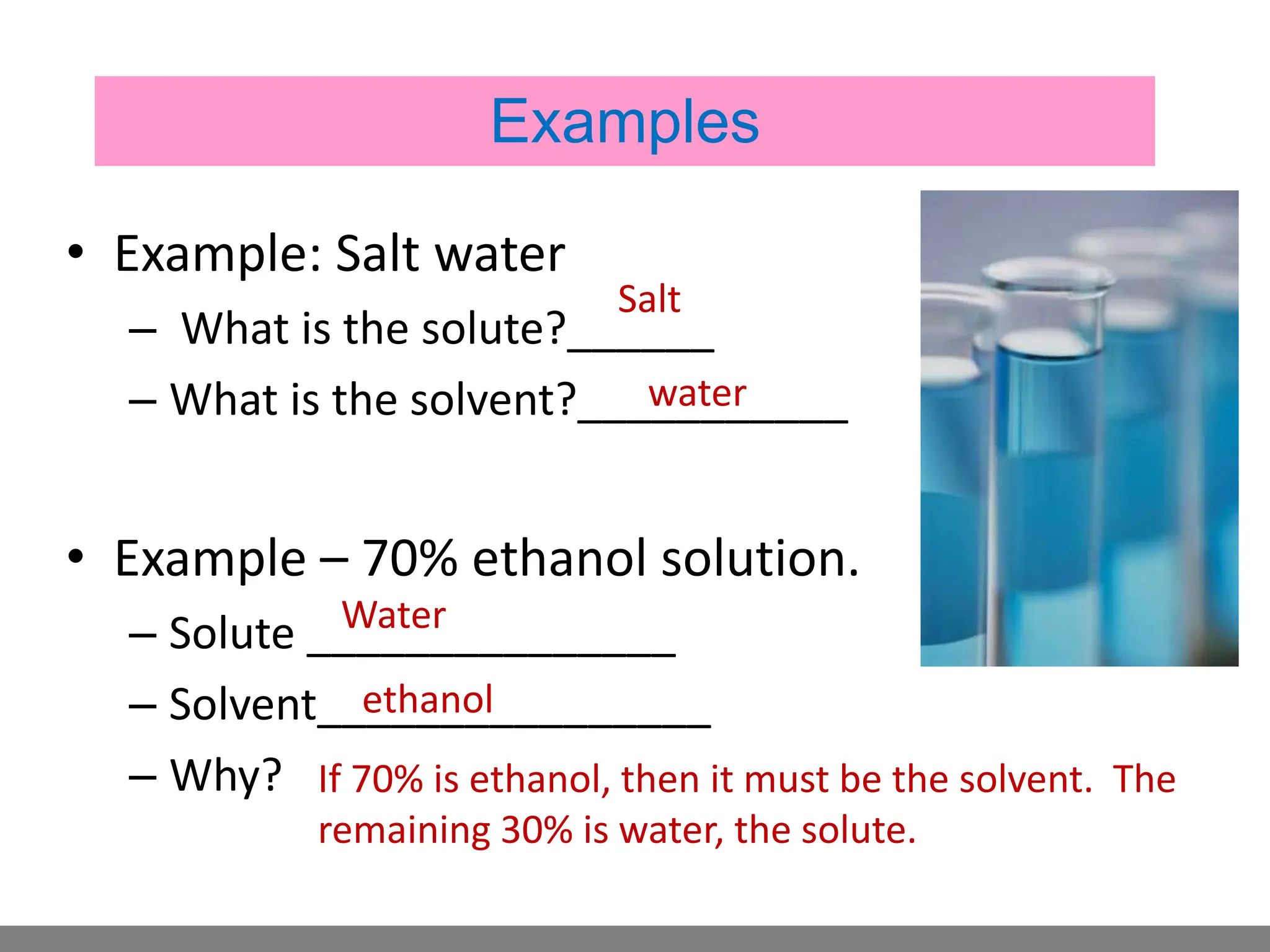
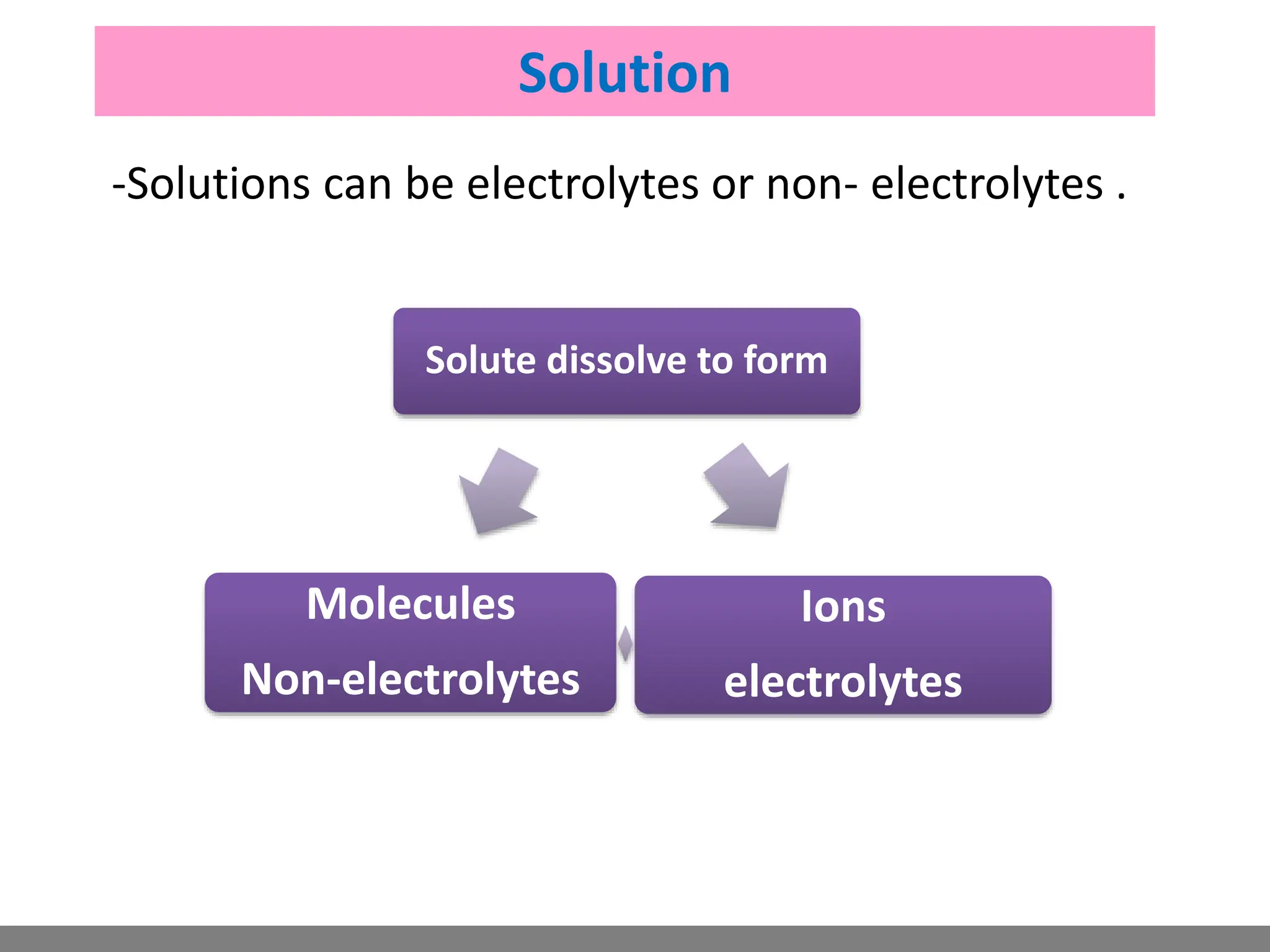
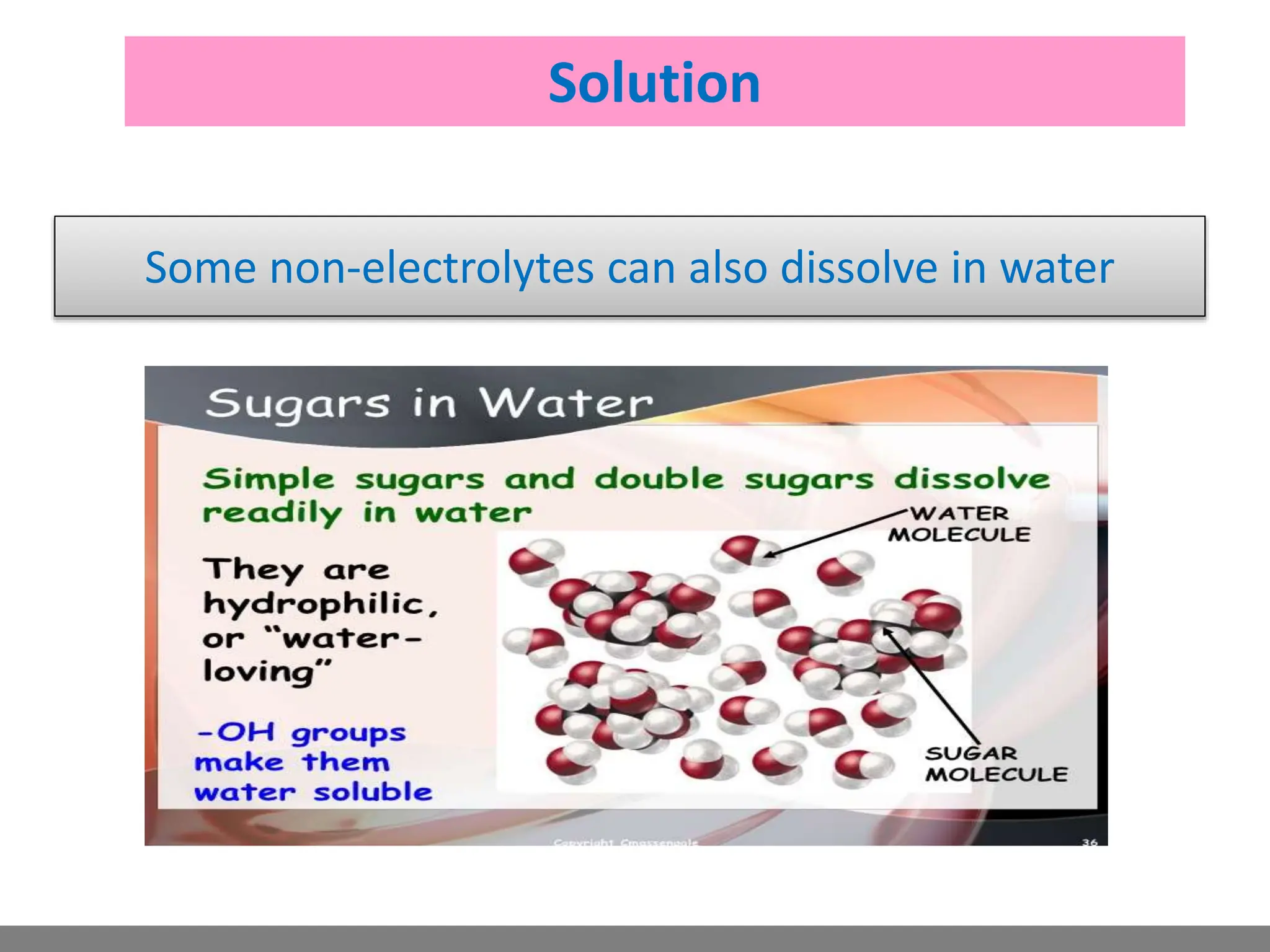
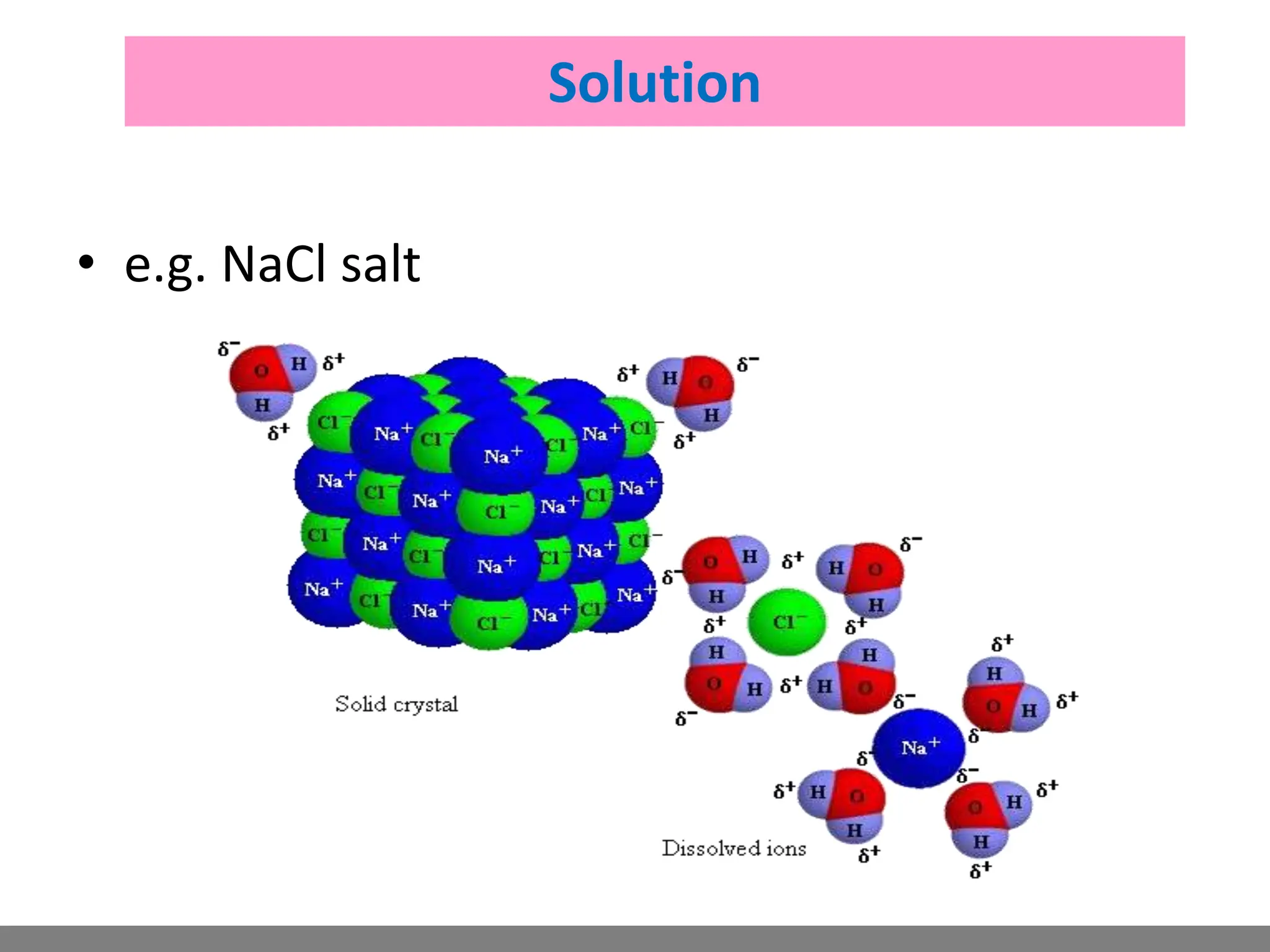
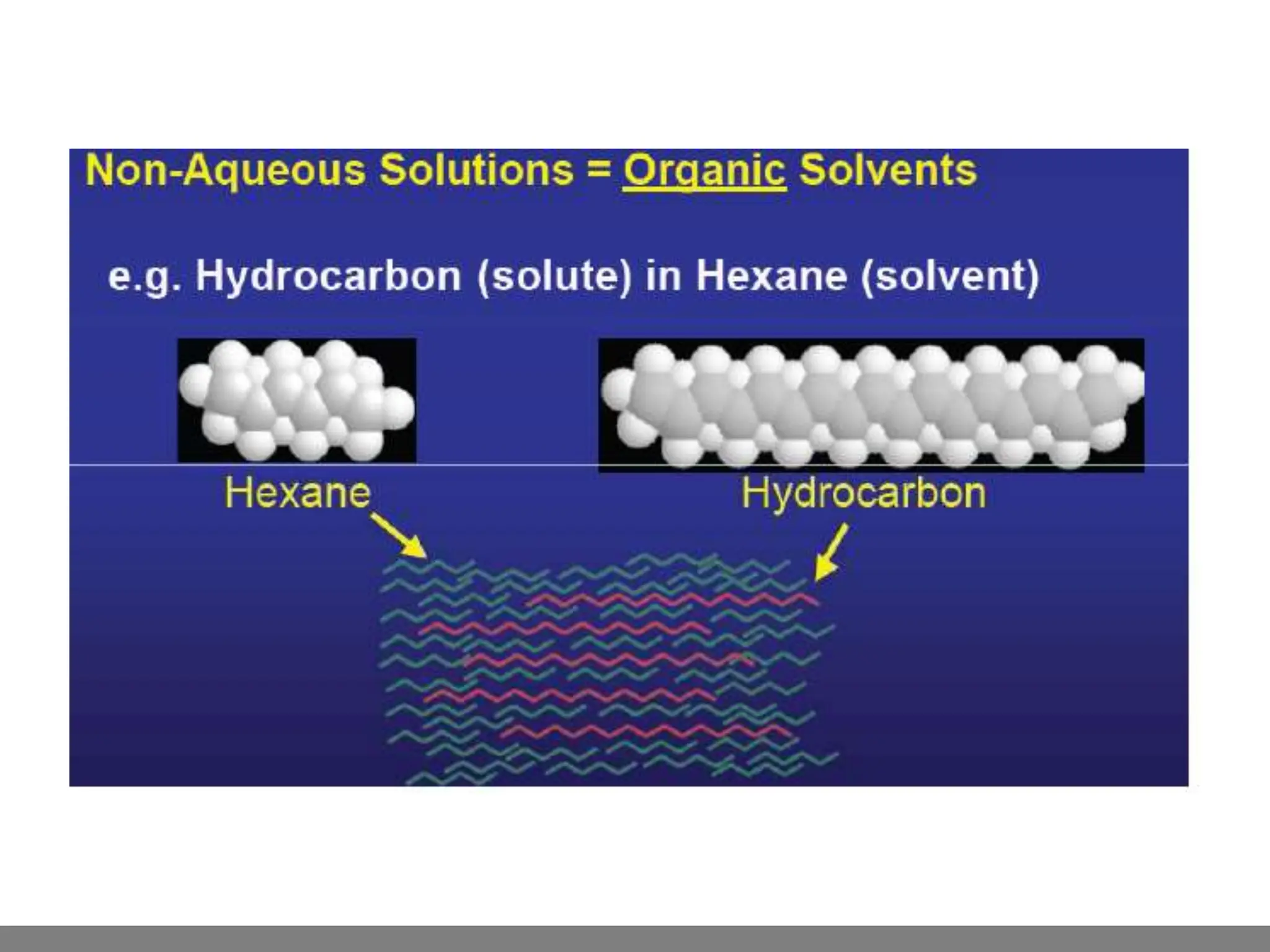
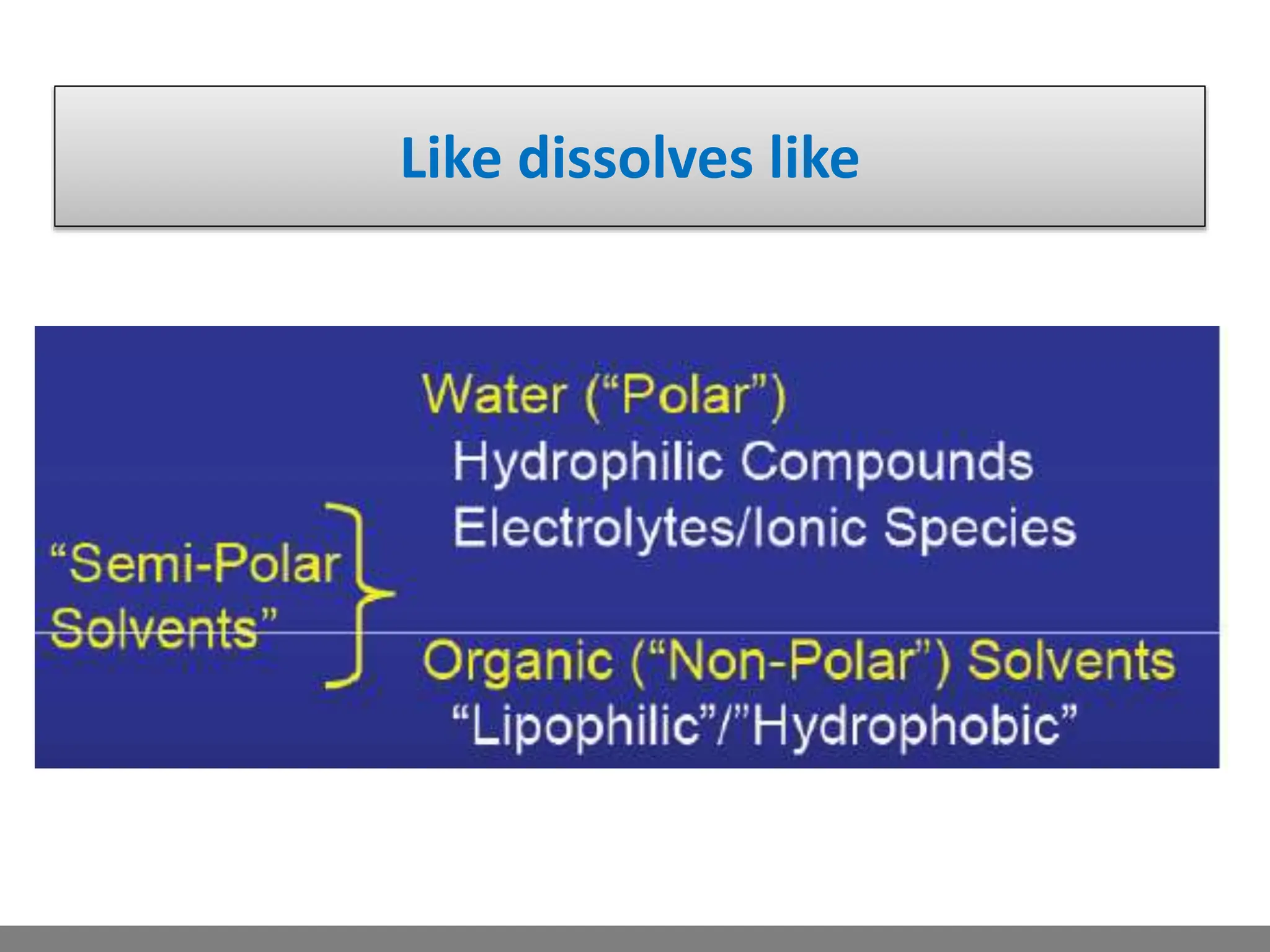
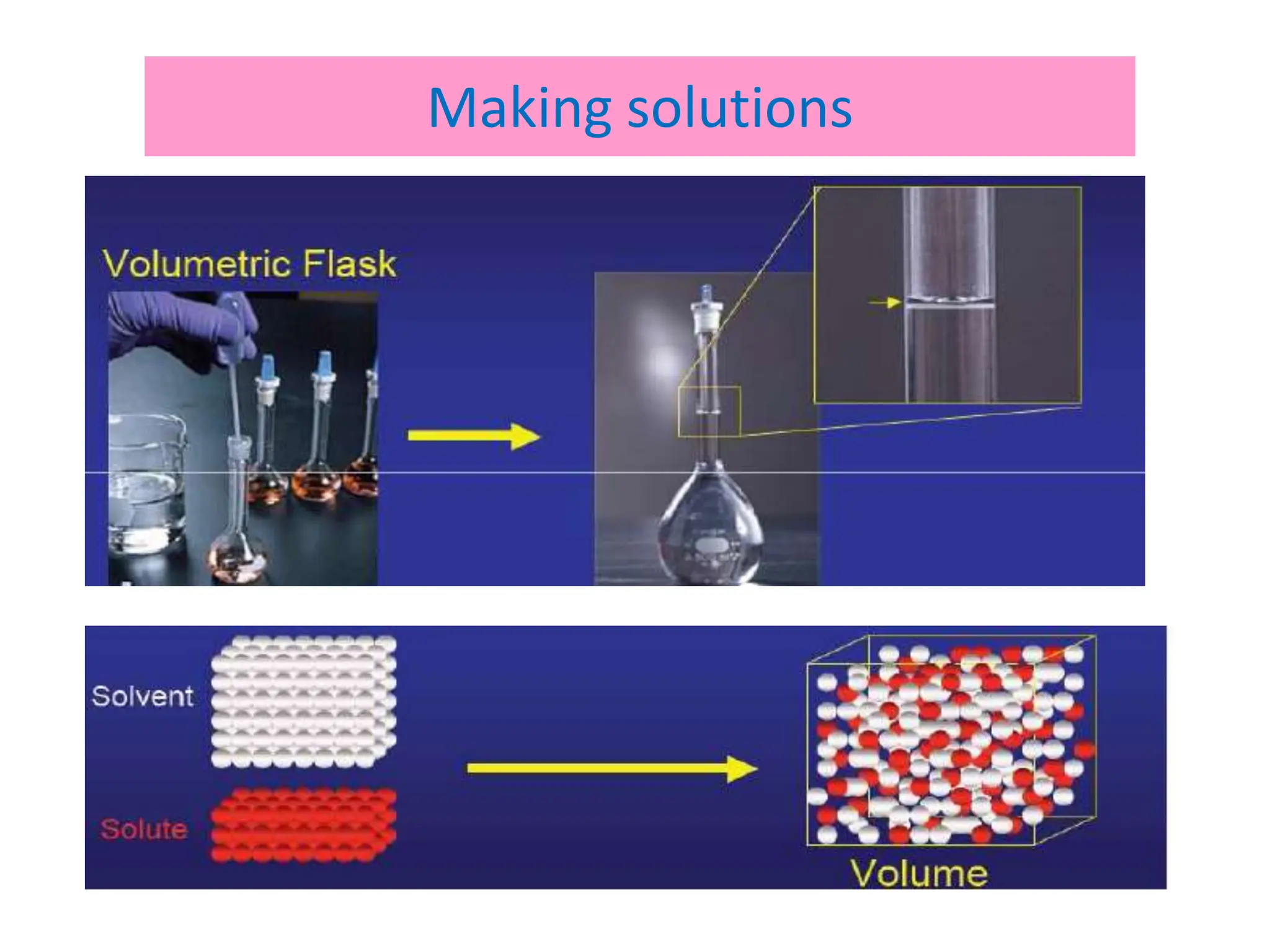
• Solution.
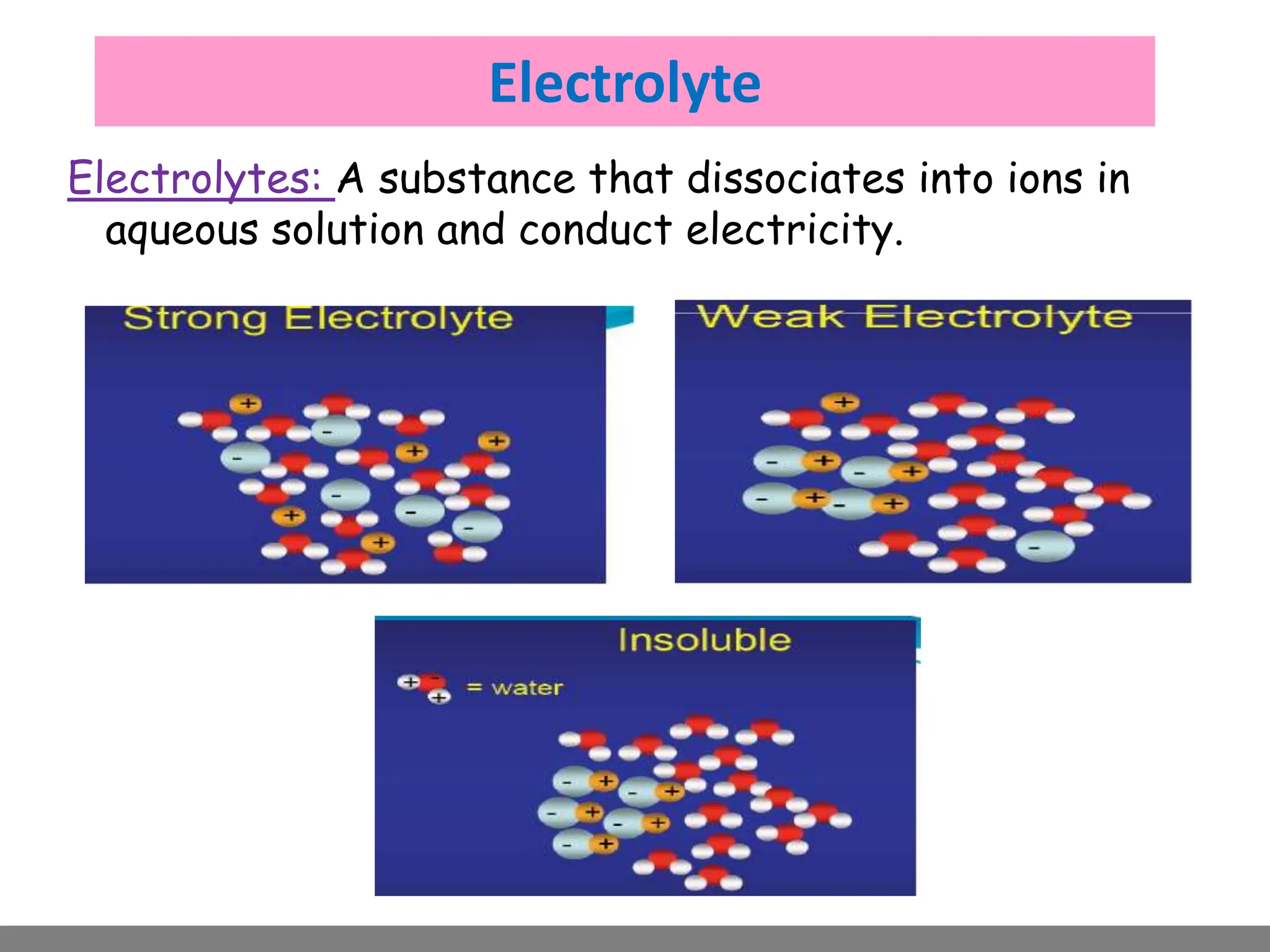
• Electrolytes.
• Solubility rules.
CHAPTER 1 BOOK OF
ANALYTICAL CHEMISTRY-
ANALYTICAL CHEMISTRY
Lecture 1 contents](https://image.slidesharecdn.com/introductiontoanalyticalchemistry-240328000610-f9521eb5/75/123123INTRODUCTIONTOANALYTICALCHEMISTRY-ppt-1-2048.jpg)