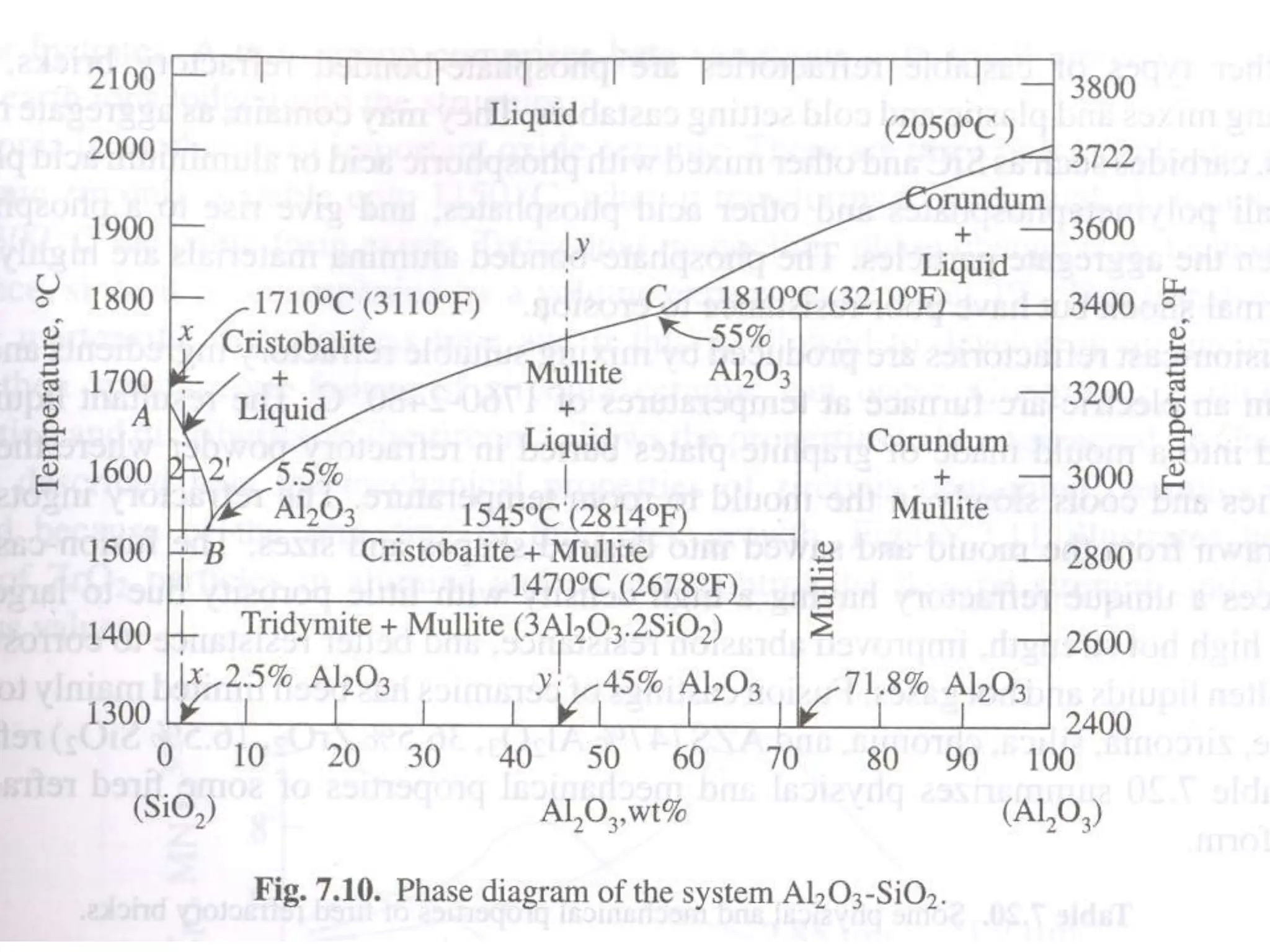
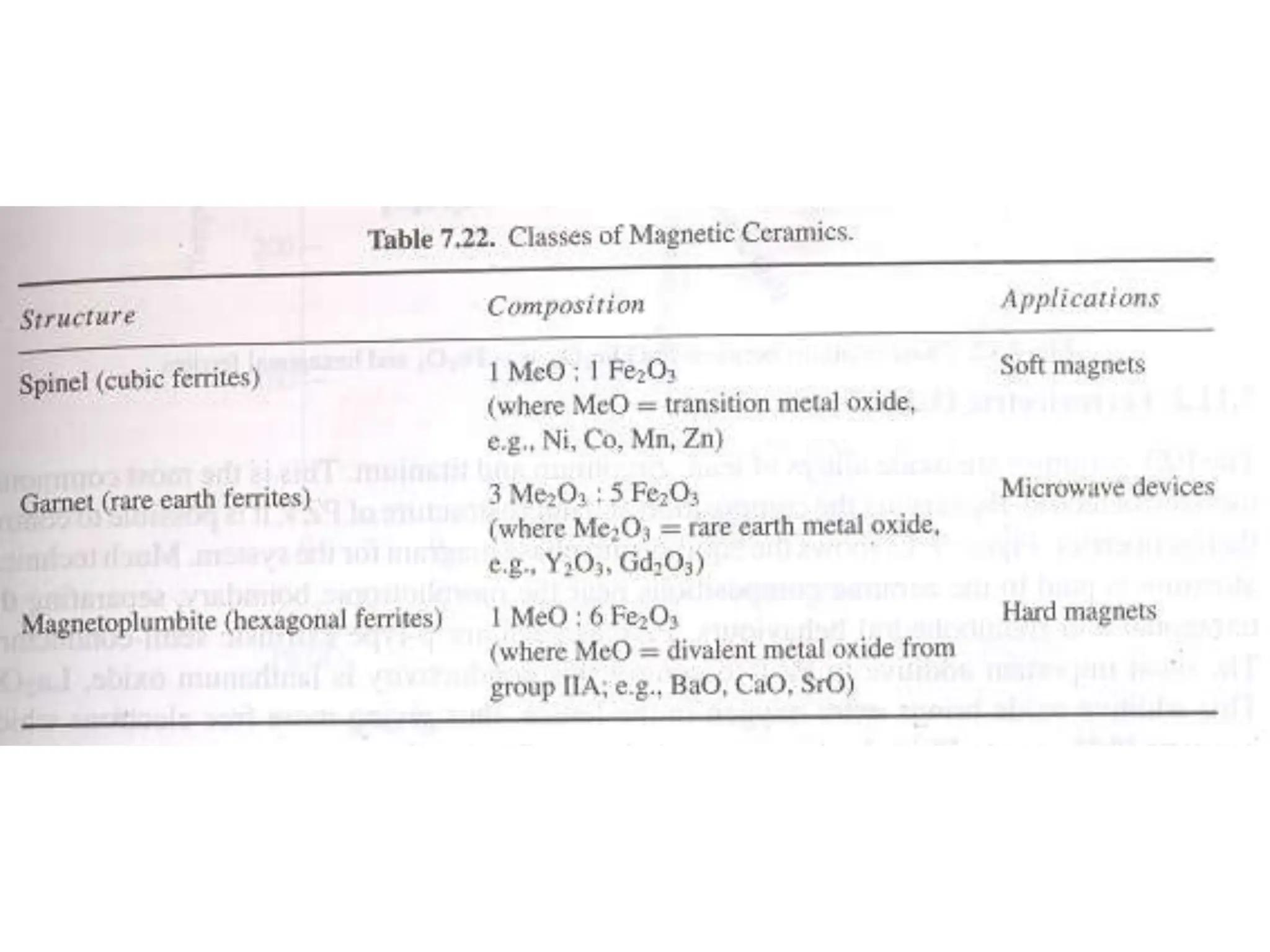
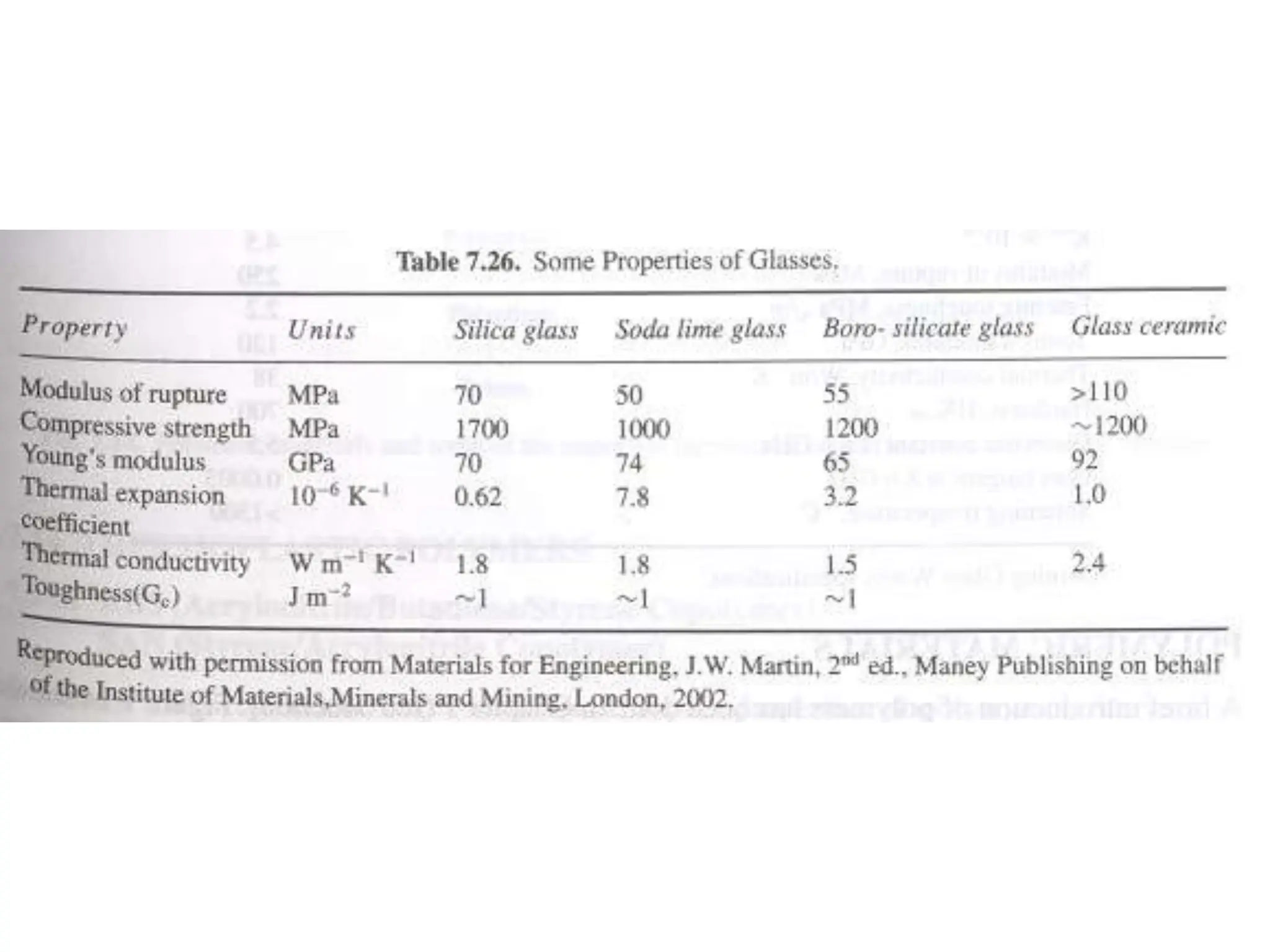
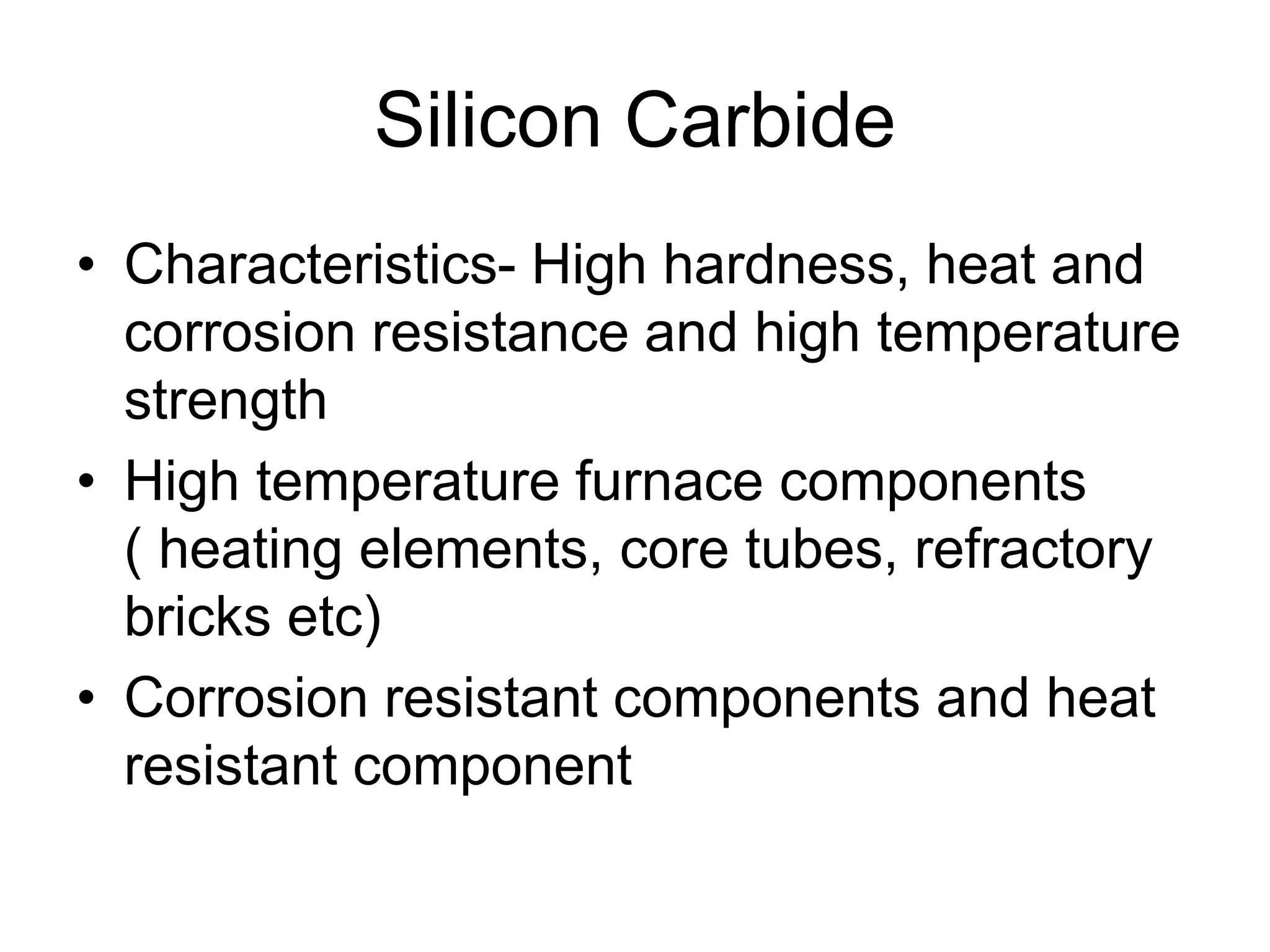
Ceramics are inorganic, non-metallic materials made by combining a metal and nonmetal. Important ceramics include silica, alumina, and clay. Ceramics have properties like hardness, heat and electrical insulation, and chemical stability. Common ceramic products are bricks, tiles, glass, ceramics, abrasives, and cements. Ceramics are brittle due to their crystal structure and imperfections. Important classes of ceramics discussed are oxide ceramics like alumina, carbides, nitrides, glasses, and non-oxide ceramics. Each type has distinct properties and applications.