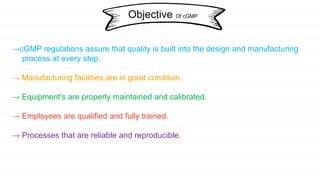
The document discusses the Current Good Manufacturing Practice (CGMP) regulations enforced by the FDA for the herbal drug industry, ensuring proper design and control of manufacturing processes. It outlines key systems such as quality management, production, laboratory control, material, packaging, and equipment that are crucial for maintaining drug safety and quality. Overall, CGMP aims to prevent contamination and errors, ensuring drug products meet regulatory standards.