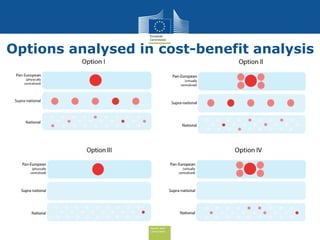
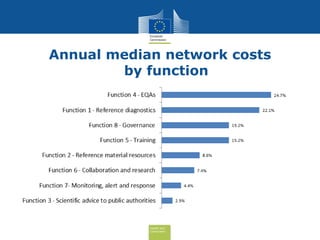
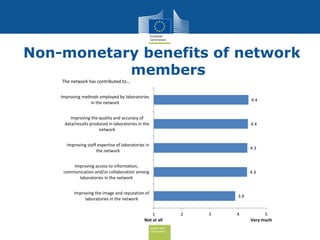
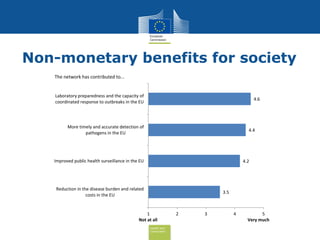
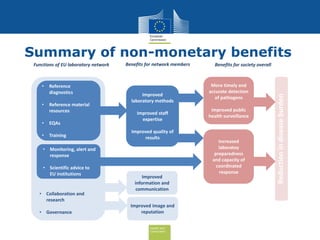
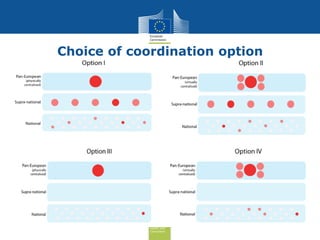
The document discusses the need for improved EU-wide provision of reference microbiology laboratories for human pathogens. It summarizes a previous study called EURLOP that evaluated options for an EU reference laboratory system but did not include a cost-benefit analysis. The current study aims to provide a cost-benefit analysis of strengthening coordination of reference microbiology in the EU. It analyzes costs and benefits of existing reference laboratory networks and finds that benefits, both monetary and non-monetary, outweigh the costs. However, issues like sustainable funding and defining the scope of networks would need addressed for a future EU reference laboratory system.