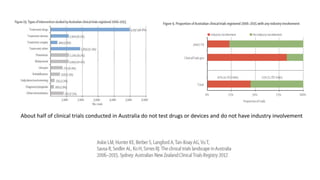
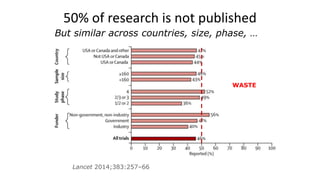
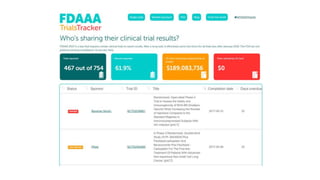
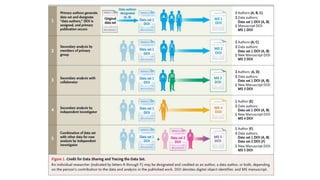
The document discusses the challenges and current state of clinical trials data sharing, highlighting that a significant portion of clinical research remains unpublished and data sharing compliance is low. It emphasizes the need for better data curation practices, common definitions of outcomes, and the creation of accessible repositories to support future data use. The document calls for improved incentives and enforcement measures to promote data sharing within the clinical research community.