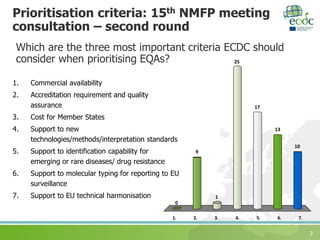
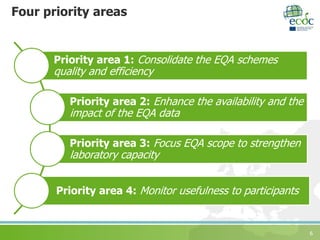
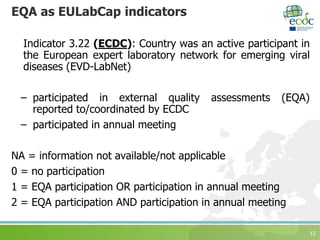
The document discusses ECDC's strategy for laboratory external quality assessment (EQA) schemes from 2017 to 2020. It notes that EQA schemes face limited resources and must adapt to new technologies like whole genome sequencing. The strategy aims to prioritize EQA schemes based on supporting new methods, emerging diseases, molecular typing, and harmonization. It sets objectives to increase country participation, improve reporting, link EQAs to capacity building, and monitor usefulness. The strategy proposes including EQA participation in the European Union Laboratory Capability Monitoring framework.