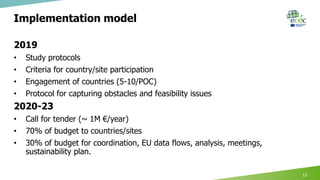
This document summarizes ECDC projects and collaborations on digital surveillance. It discusses two proof-of-concept studies: 1) Surveillance of pan-drug resistance which would use laboratory and electronic health record data through existing networks like WHONet. 2) Surveillance for priority diseases using electronic health records to describe cases with more complete risk factor data than currently available. The document outlines opportunities and challenges for these studies and proposes a multi-year implementation model to engage countries, address obstacles, and establish sustainable EU data flows and analyses.