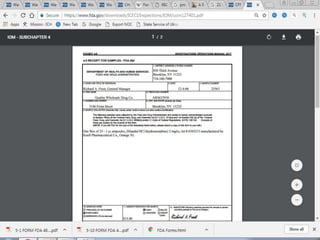
The document defines FDA warning letters and describes the process of FDA inspections that can lead to warning letters. It explains that warning letters notify companies of violations found during inspections and investigations. Companies must promptly correct issues and FDA will check that corrections are adequate. The document also describes different types of warning letters for various regulated industries and how to browse existing warning letters on the FDA website.