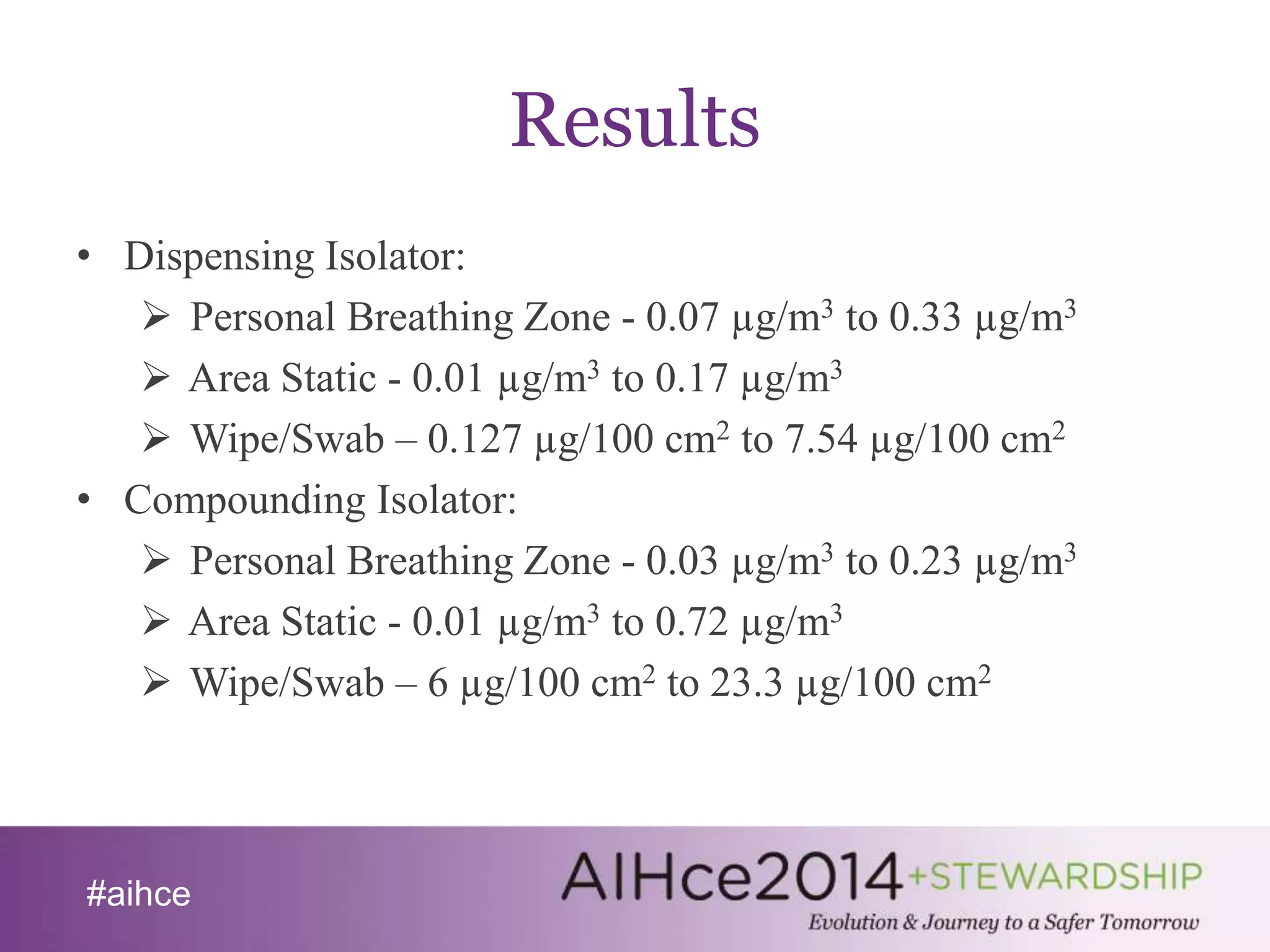
The document discusses the significance of particulate containment validation in reducing pharmaceutical exposure, particularly in India's rapidly growing contract manufacturing sector. It highlights challenges such as inadequate risk assessment, lack of occupational exposure limits, and insufficient analytical methods, while presenting a validation procedure that involved monitoring four isolators. Results showed that exposure levels were mostly below the containment performance target, but surface contamination exceeded thresholds, leading to key recommendations for improving containment practices.