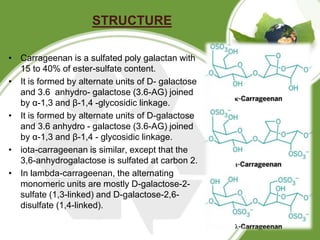
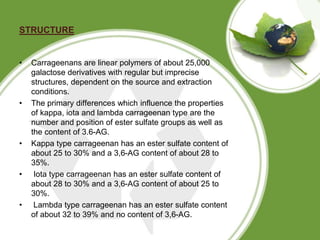
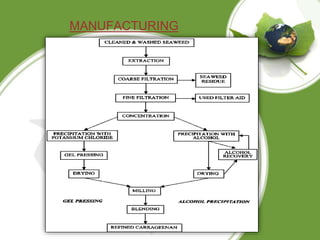
This document discusses carrageenan, which is extracted from red seaweed. It exists in three main varieties - kappa, iota, and lambda - which differ in their sulfate content. Carrageenan has linear sulfated polysaccharide structure and is used widely in food as a gelling, thickening, and stabilizing agent. It is manufactured by washing, alkali treatment, and precipitation of seaweed. Its properties include solubility, gelling, viscosity, stability, and interaction with other components. It has various applications in food products as well as medical and other industrial uses.