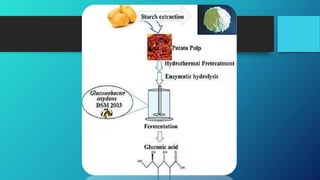
The document discusses the industrial production of gluconic acid. It begins by introducing gluconic acid and describing its microbial production process using fungi like Aspergillus niger or bacteria such as Gluconobacter oxydans. The history of gluconic acid production dating back to the 1870s is then summarized. The document proceeds to discuss the enzymatic reactions involved in gluconic acid formation, fermentation processes, production of pure gluconic acid, recovery methods, a flowchart of the production process, and various uses of gluconic acid and its derivatives in industries like food, pharmaceuticals, detergents, and more.