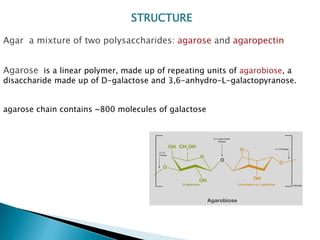
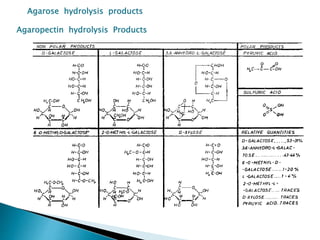
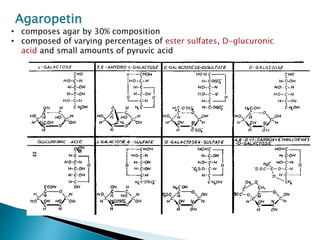
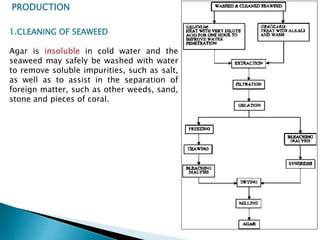
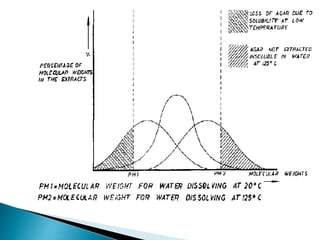
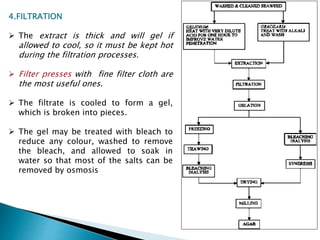
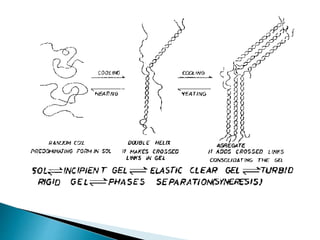
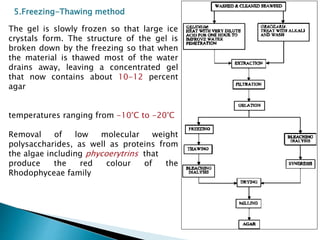
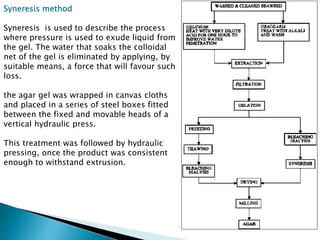
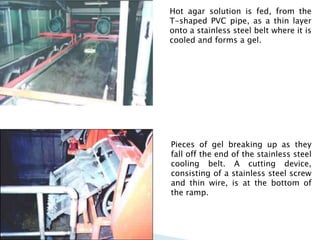
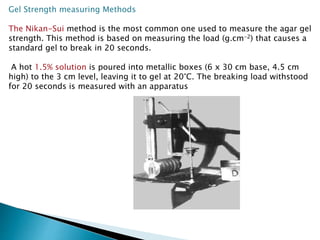
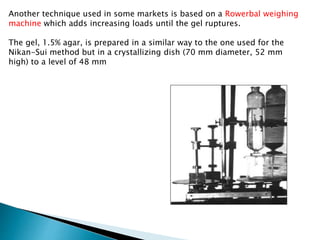
Agar is a polysaccharide extracted from red seaweeds that is used widely in food applications due to its gelling properties. It forms thermo-reversible gels when dissolved in boiling water and cooled. The document details the production process of extracting and purifying agar from seaweeds. It also describes agar's properties including its solubility, viscosity, stability at different pH levels and temperatures. Finally, the summary discusses agar's many uses in foods such as confectionaries, bakery products, meat and dairy applications due to advantages like its wide pH range and ability to gel without adding other ingredients.