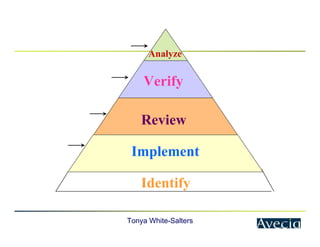
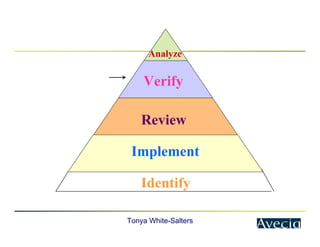
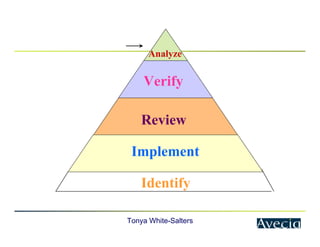
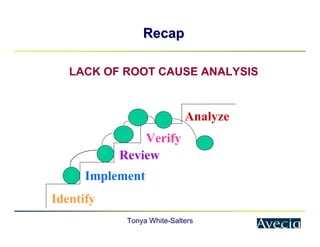
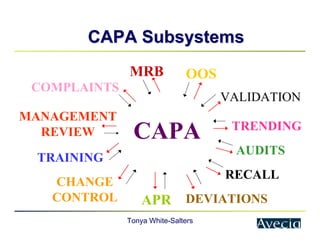
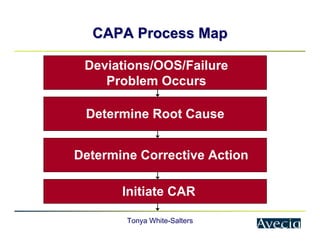
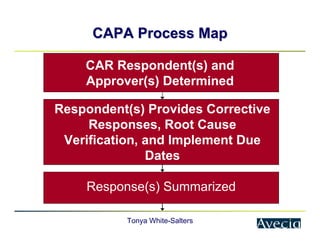
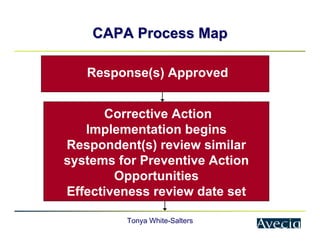
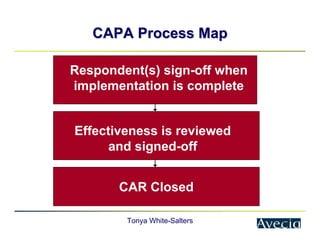
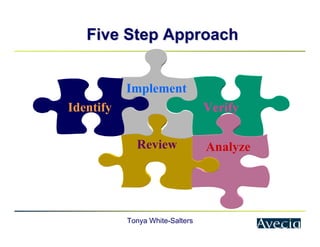
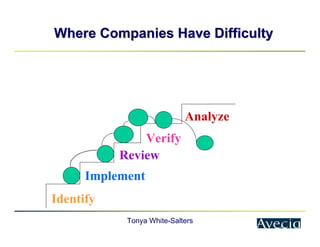
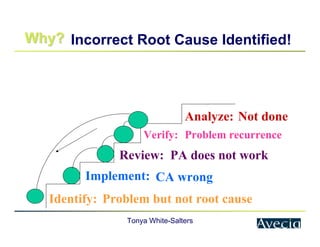
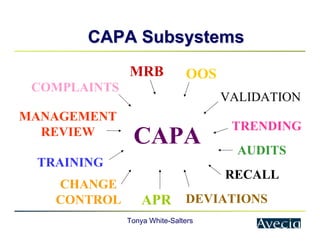
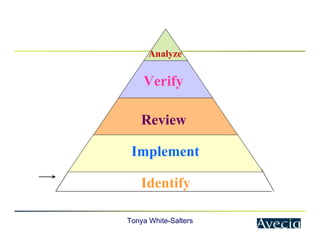
The document outlines a five-step approach to Corrective and Preventive Actions (CAPA) in compliance with FDA regulations, detailing the processes for identifying root causes, implementing corrective actions, and verifying effectiveness. It emphasizes the importance of thorough investigations into nonconformities and provides examples of citations related to failures in CAPA implementation. Additionally, it highlights common difficulties companies face in executing CAPA processes and the need for systematic procedures to address quality issues.














![Tonya White-Salters
Example Citation
Example Citation -
- CBER
CBER
• Failure to establish and maintain adequate
procedures for implementing corrective
and preventive action, as required by 21
CFR 820.100(a) and (b). For-example:
• (a) The procedure titled corrective Action
Handling [redacted] was not approved and
implemented to address corrective and
preventive action and no established
procedure was found to have been in
place.](https://image.slidesharecdn.com/capa-240713022314-1d9b07fe/85/Corrective-and-Preventive-Action-blueprint-pdf-26-320.jpg)