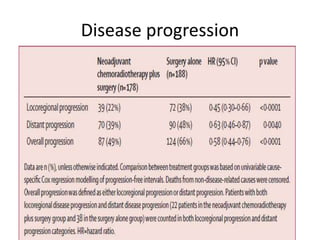
This randomized controlled trial compared neoadjuvant chemoradiotherapy plus surgery to surgery alone in 368 patients with resectable esophageal or junctional cancer. Patients receiving neoadjuvant treatment had significantly improved overall survival (48.6 vs 24 months) and progression-free survival (37.7 vs 16.2 months). R0 resection rates were also higher in the neoadjuvant group (92% vs 69%). The trial demonstrated that preoperative chemoradiotherapy improves long-term outcomes for esophageal cancer patients.










![Initial results in 2012
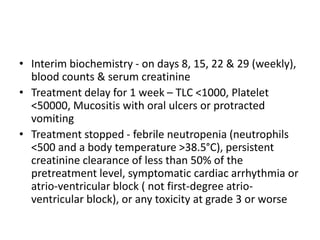
• Minimum follow-up of 24 months (median
follow-up 45 months [range 25.2–80.9, IQR 32.6–
60.6])
• Absolute benefit in 5-year overall survival in
favour of the multimodality group
• Treatment completion rate 95%
• Rate of occurrence of grade 3 adverse events 17%
• R0 resection – 92% in the multimodality group
compared with 69% surgery alone group
(p<0.001)](https://image.slidesharecdn.com/crosstrial-160126184406/85/Cross-trial-22-320.jpg)



![Results for overall survival
Median overall
survival
NACRT f/b surgery Surgery alone Stastical
significance
overall 48.6 months 24.0 months HR 0.68 with 95%
CI, range 0.53–0.88]
squamous cell
carcinomas
81.1 months 21.1 months HR 0.48 [95% CI,
range 0.28–0.83])
adenocarcinomas 43.2 months 27.1 months HR 0.73 [95% CI,
range 0.55 – 0.98
At 1 years 81% 70% HR 0.57 [95% CI,
range 0.37 – 0.88]
At 2 years 67% 50% HR 0.59 [CI 95%,
range 0.43 – 0.82]
At 3 years 58% 44% HR 0.65 [CI 95%
0.49 – 0.88]
At 5 years 47% 33% HR 0.67 [CI 95%
0.51 – 0.87]](https://image.slidesharecdn.com/crosstrial-160126184406/85/Cross-trial-26-320.jpg)
![Results for progression free survival
Median
progression free
survival
NACRT f/b surgery Surgery alone Stastical
significance
overall 37.7 months 16.2 months HR 0.64 [95% CI,
range 0.49 – 0.82]
squamous cell
carcinomas
74.7 months 11.6 months HR 0.48 [95% CI,
range 0.28 – 0.82
adenocarcinomas 29.9 months 17.7 months HR 0.69 [95% CI,
range 0.52 – 0.92]
At 1 years 71% 54% HR 0.55 [95% CI,
range 0.39 – 0.77
At 2 years 60% 41% HR 0.57 [95% CI,
range 0.42 – 0.77]
At 3 years 51% 35% HR 0.62 [CI 95%,
range 0.47–0.82]
At 5 years 44% 27% HR 0.61 [CI 95%,](https://image.slidesharecdn.com/crosstrial-160126184406/85/Cross-trial-27-320.jpg)