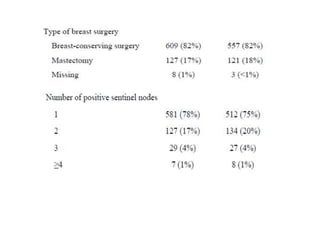
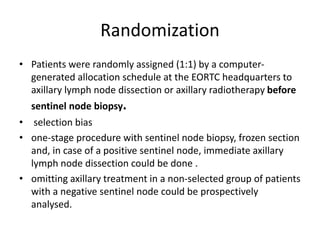
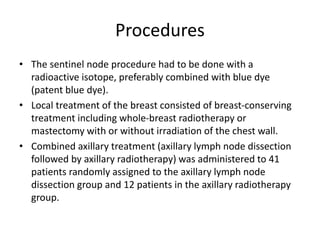
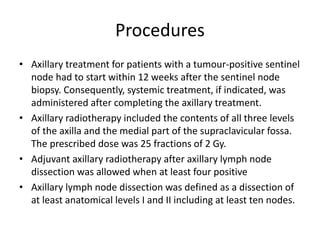
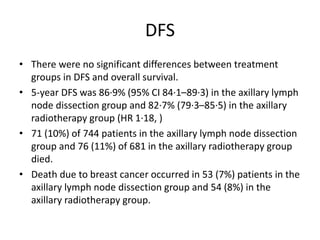
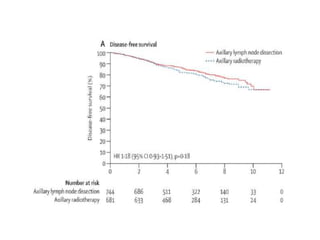
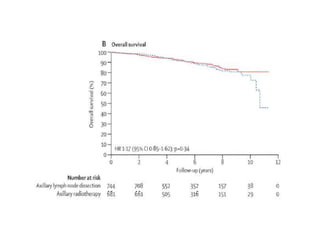
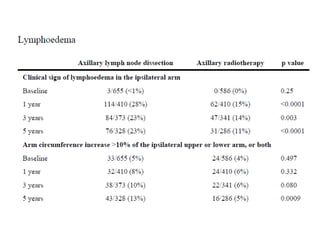
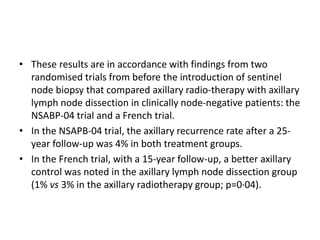
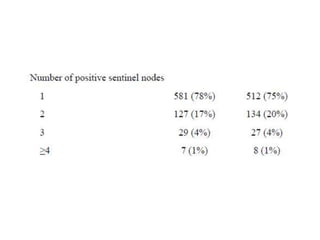
Axillary radiotherapy provides comparable regional control to axillary lymph node dissection for breast cancer patients with positive sentinel nodes, with fewer side effects. The AMAROS trial found similar 5-year axillary recurrence, disease-free survival, and overall survival between the radiotherapy and dissection groups. However, radiotherapy resulted in significantly less lymphedema. While control was excellent with both treatments, the trial was underpowered to definitively show non-inferiority due to lower-than-expected axillary recurrences.