This document provides an overview of stomach cancer including its epidemiology, risk factors, pathology, clinical presentation, staging, treatment and outcomes. Some key points:
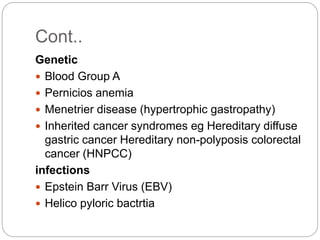
- Stomach cancer is the 4th most common cancer and 2nd leading cause of cancer death worldwide. Risk factors include H. pylori infection, diet low in fruits/veggies, smoking, and family history.
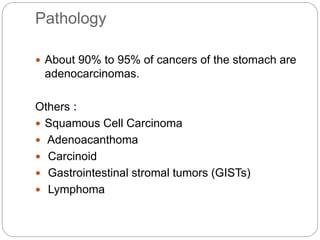
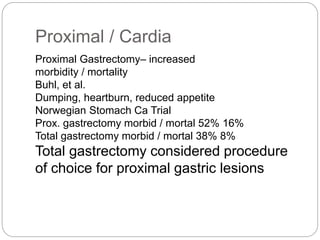
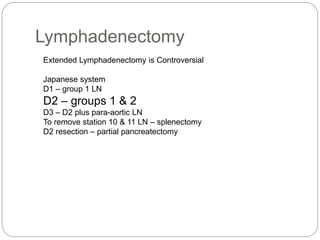
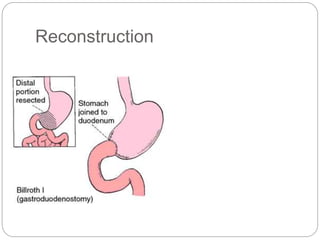
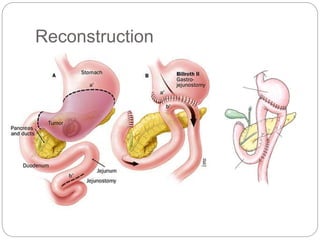
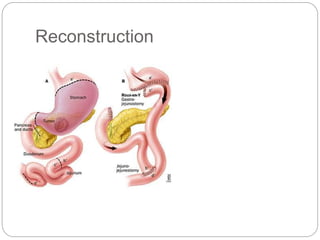
- Most stomach cancers are adenocarcinomas. Treatment involves surgical resection of the tumor along with lymph nodes, with or without chemotherapy or radiation before/after surgery depending on staging.
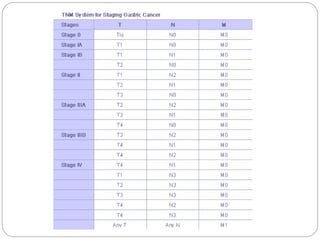
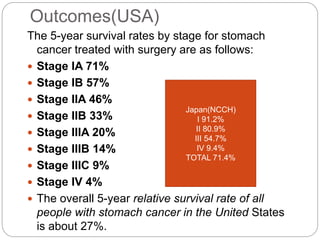
- 5-year survival ranges from 71% for early stage to 4% for late stage disease. Outcomes are generally better in Asia