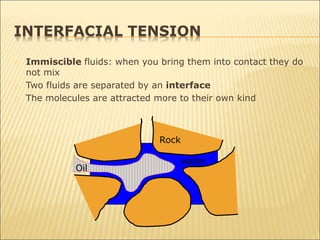
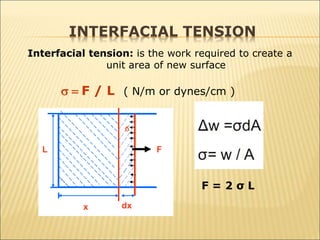
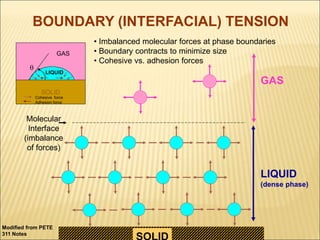
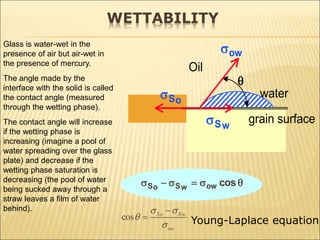
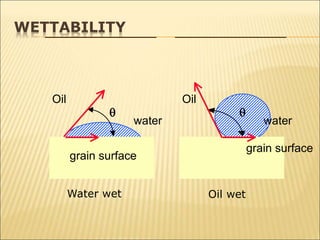
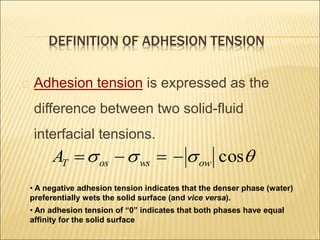
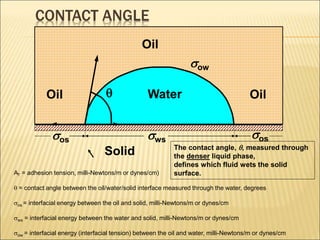
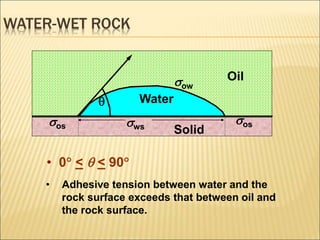
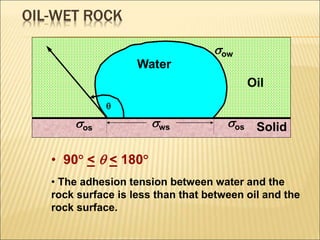
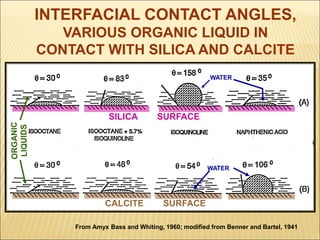
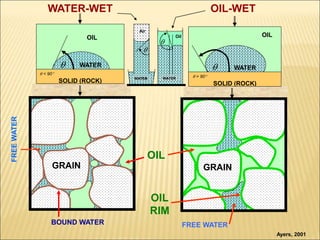
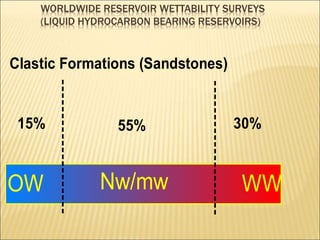
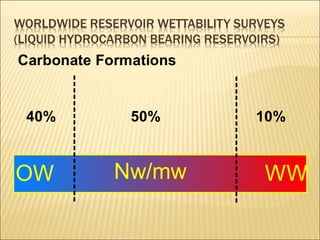
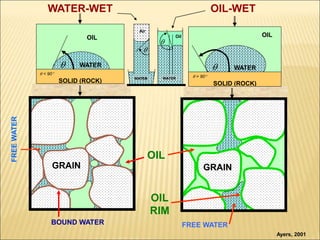
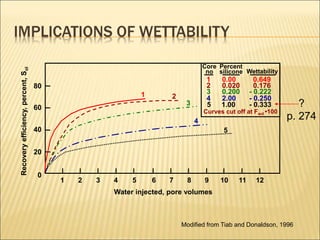
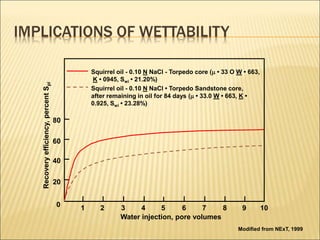
When two immiscible fluids such as oil and water are present in rock pores, interfacial tension arises at the boundary between the fluids due to imbalanced molecular forces. Wettability refers to whether the rock preferentially interacts with and spreads one fluid over the other. It is determined by measuring the contact angle between the fluids and solid surface. Wettability affects fluid distributions and flow properties in the reservoir, with water-wet rocks typically yielding more oil during waterflooding recovery than oil-wet rocks.