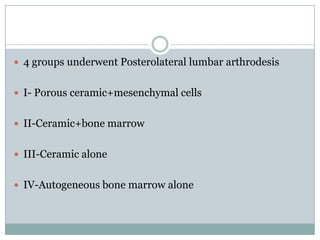
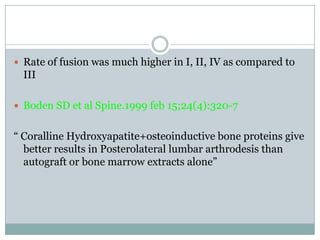
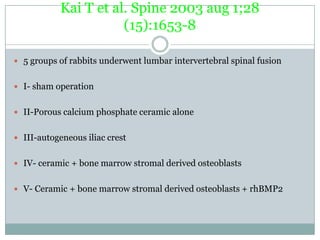
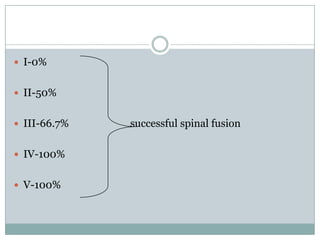
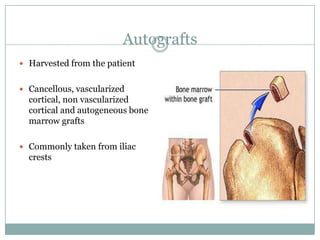
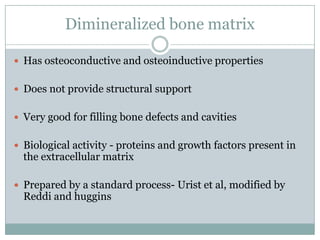
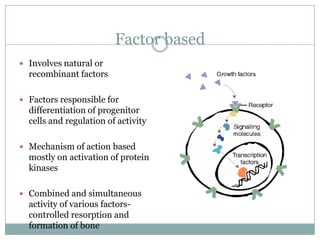
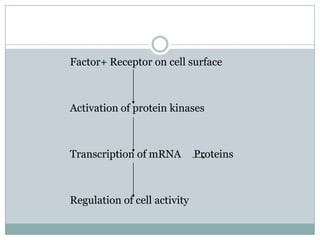
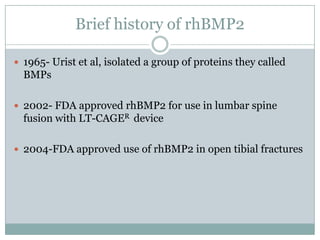
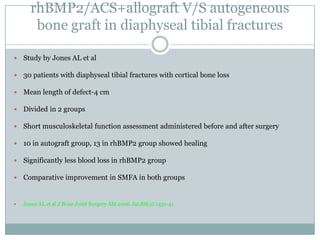
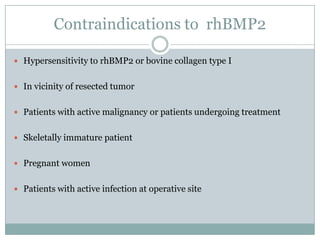
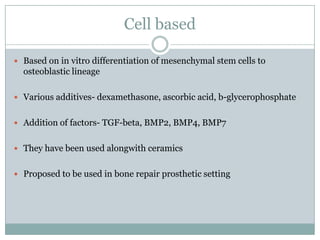
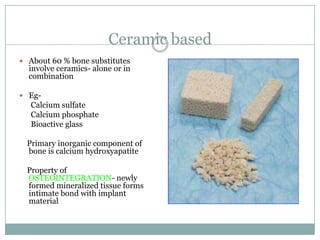
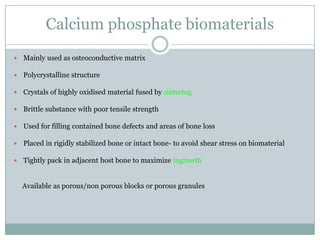
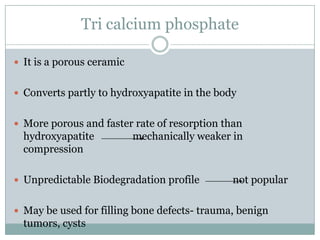
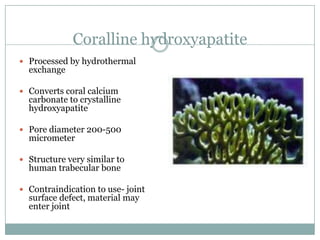
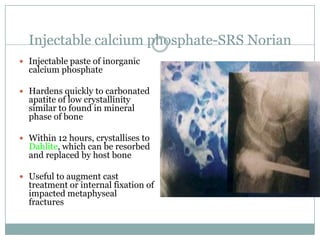
Dr. Paudel discussed bone graft substitutes, which are used to fill bone defects and promote healing. They discussed various types including allografts, ceramics, polymers, and composites. Allografts have disadvantages like immune reactions and disease transmission. Ceramics are osteoconductive but not structural. Composites combining materials like ceramics, cells, and growth factors may provide better fusion than any component alone. The ideal bone graft substitute would be osteoconductive, osteoinductive, and provide structural support like autografts, but without their disadvantages.












![ Studies have been done in cases of impacted extra articular
distal end radius fractures with good results
Jupiter et al. J Orthop Trauma 1997;11:110-6
Kopylov et al. J Hand Surg [Br]. 1996;21:768-71
Kopylov et al. Acta Orthop Scand 1999;70;1-5](https://image.slidesharecdn.com/bonegraftsubstitutes-130617105756-phpapp01/85/Bone-graft-substitutes-45-320.jpg)
![Norian SRS in radial osteotomies
study by Logano calderon et al
Retrospective analysis of 6 elderly patients with corrective radial
osteotomies
Fixed with angular stable implants+ Norian SRS
All osteotomies healed
Post op DASH-28 points, Modified Mayo score-68
Logan calderon et al J Hand Surg[Am] 2007 sep;32(7):976-83](https://image.slidesharecdn.com/bonegraftsubstitutes-130617105756-phpapp01/85/Bone-graft-substitutes-46-320.jpg)