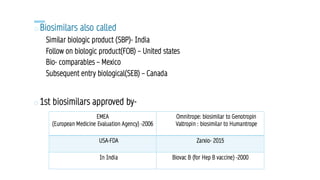
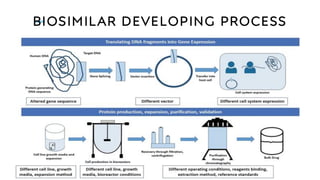
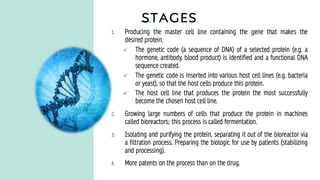
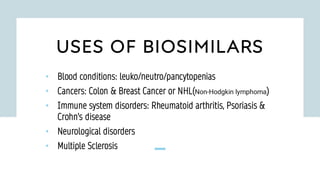
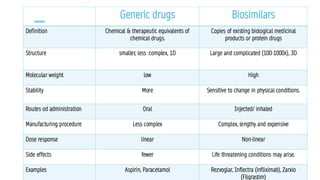
The document discusses biosimilars, which are bio-therapeutic products similar in quality, safety, and efficacy to reference biologics, and outlines their development processes, uses, advantages, and limitations. It also covers the global regulatory landscape for biosimilars, highlighting the approval pathways in India and the necessity for robust regulations. Key points include the distinction between generic drugs and biosimilars, as well as the potential benefits and concerns regarding biosimilars in clinical settings.