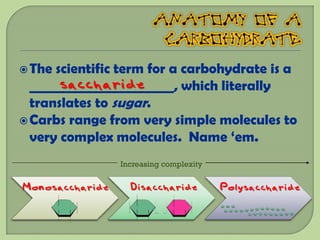
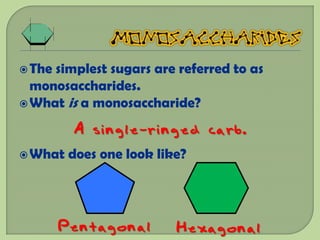
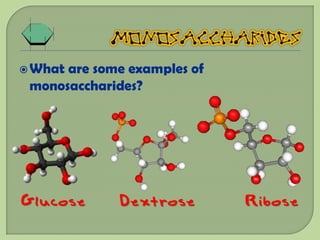
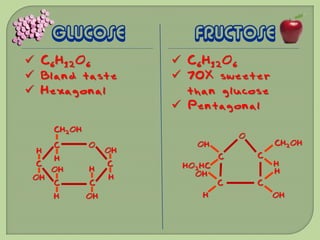
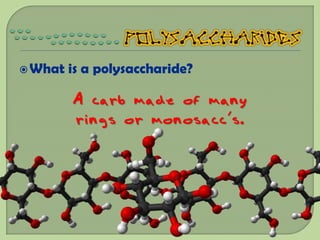
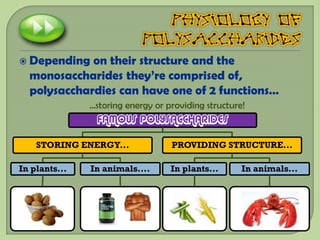
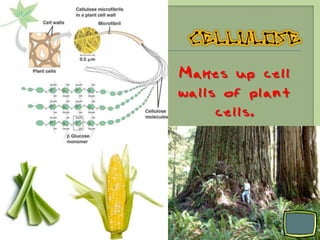
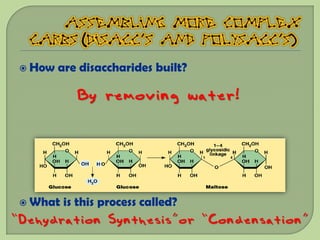
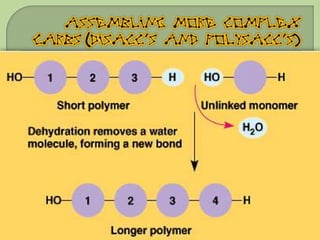
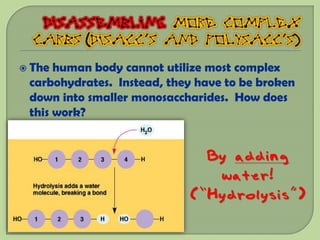
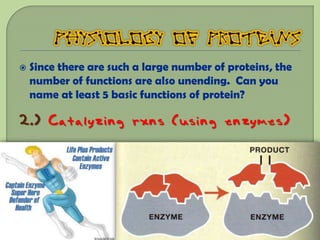
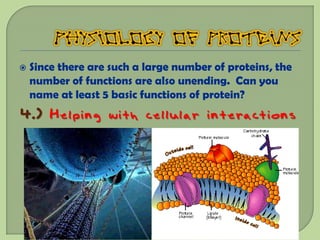
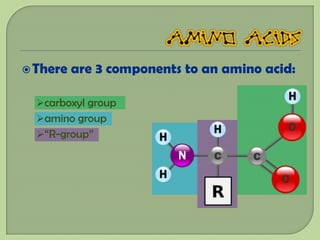
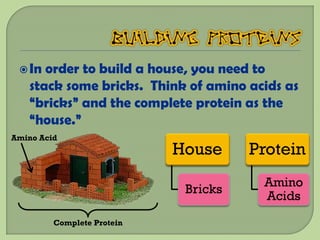
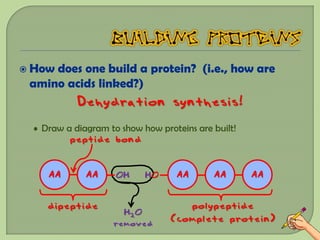
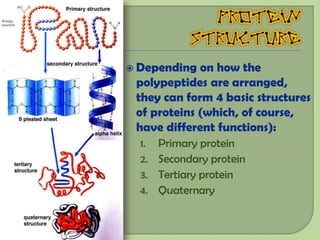
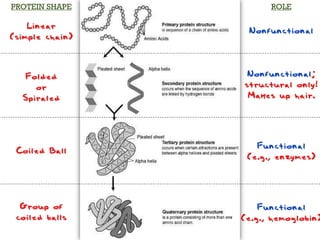
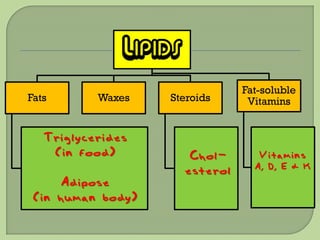
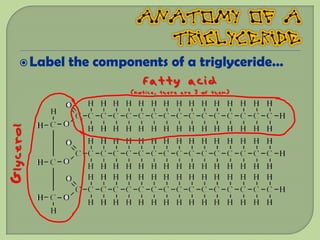
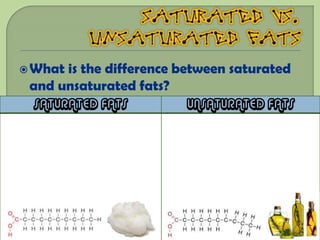
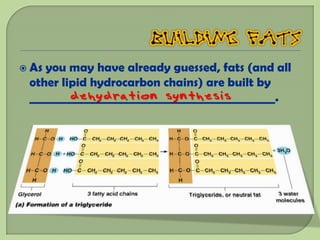
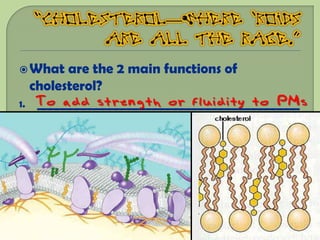
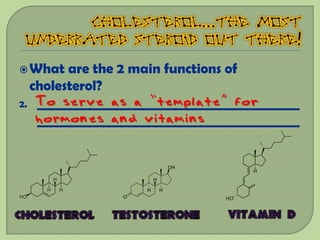
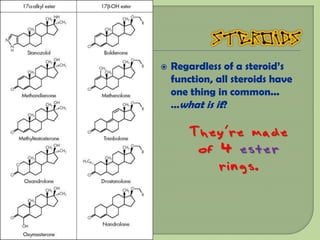
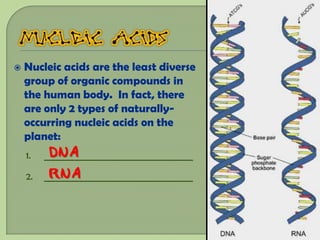
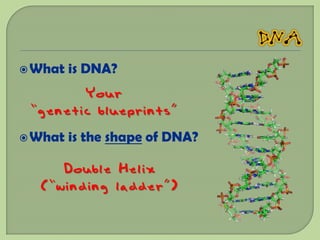
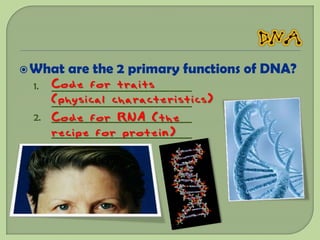
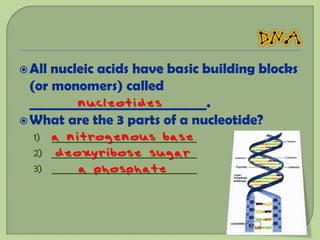
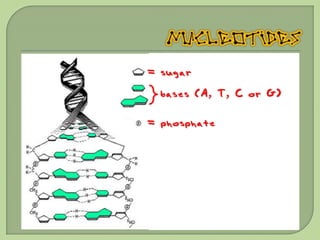
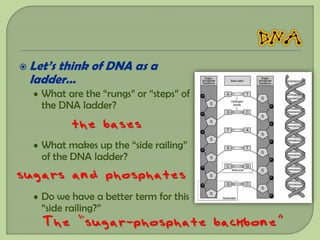
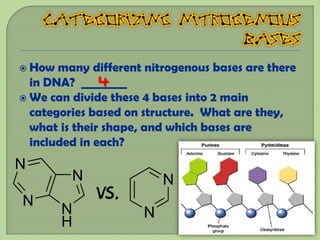
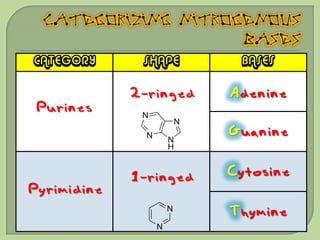
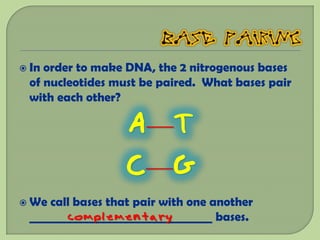
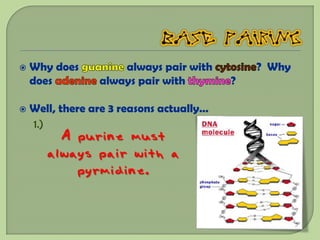
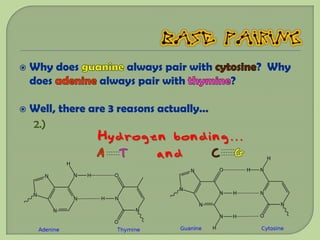
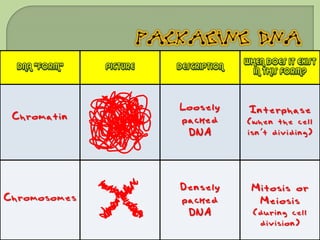
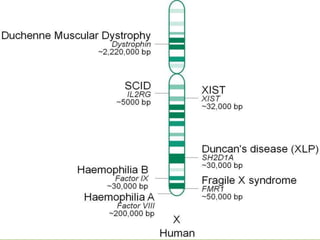
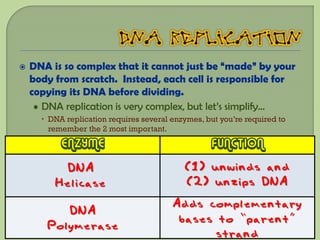
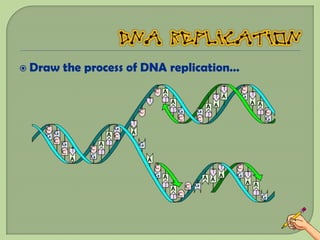
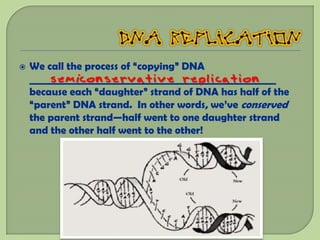
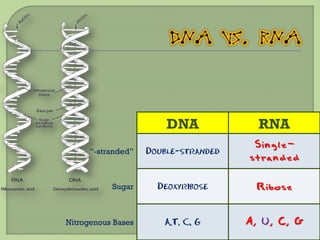
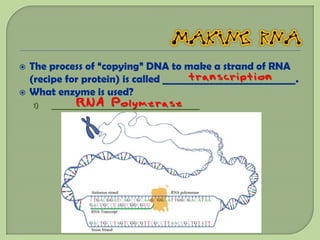
There are 4 main macromolecules that make up living things: carbohydrates, proteins, lipids, and nucleic acids. Carbohydrates include sugars and starches and are made up of carbon, hydrogen, and oxygen. They serve functions like energy storage. Proteins are made of amino acids and perform roles such as building muscle. Lipids include fats and cholesterol and contain hydrocarbon chains. Nucleic acids include DNA and RNA and contain nucleotides that code for traits and proteins. The document provides detailed information about the structures, functions, and examples of these four important macromolecule groups.