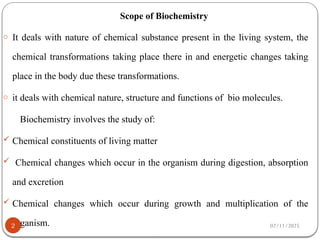
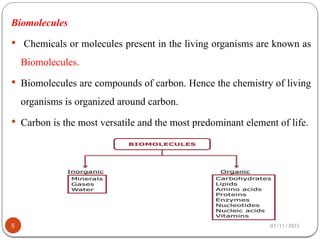
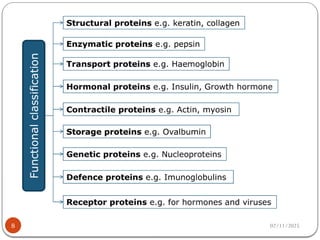
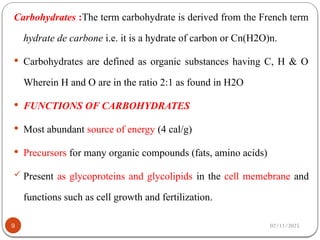
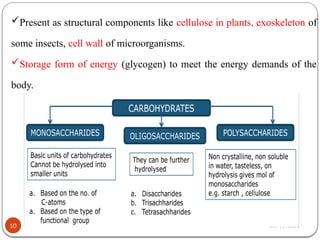
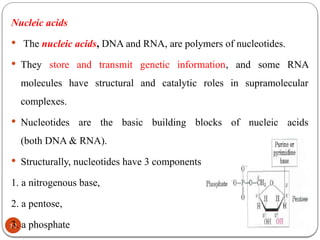
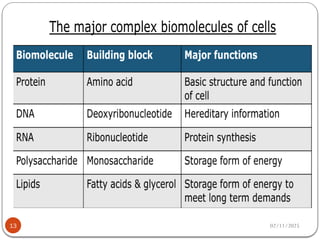
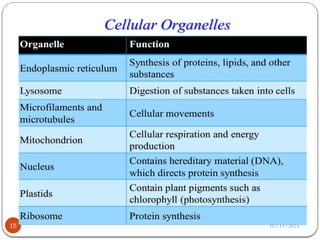
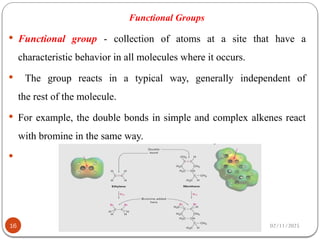
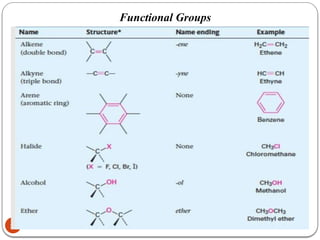
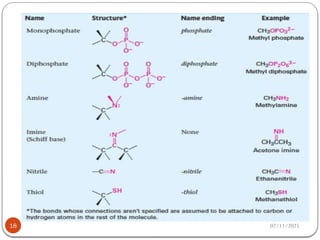
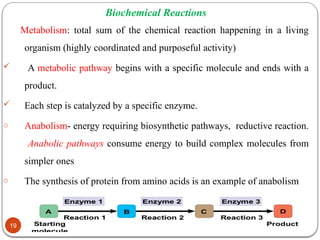
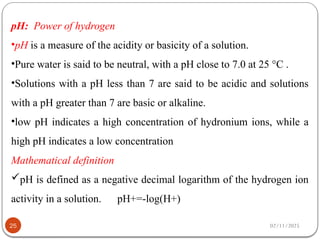
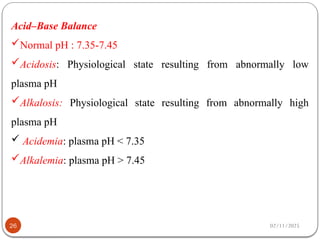
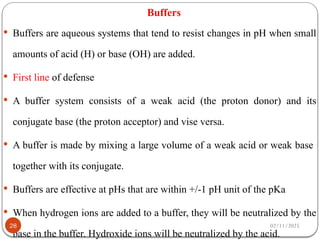
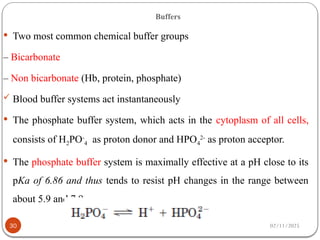
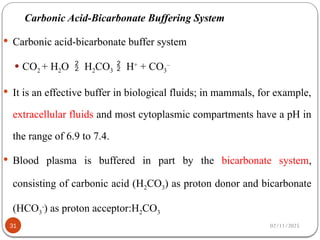
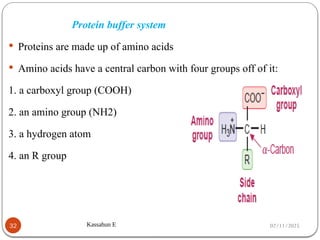
Biochemistry is the study of the chemical composition and changes in living matter, focusing on the structure and interactions of complex molecules within cells. It plays a vital role in medicine, pathology, nutrition, and understanding diseases through biochemical alterations. Major biomolecules include proteins, carbohydrates, lipids, and nucleic acids, which are critical for cellular structure, energy storage, and genetic information transmission.