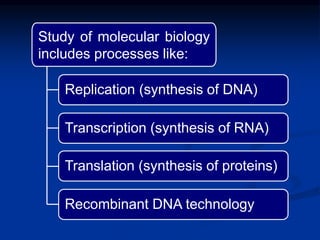
1. Living organisms are composed of biomolecules like nucleic acids, proteins, carbohydrates and lipids made from carbon, hydrogen, oxygen and nitrogen.
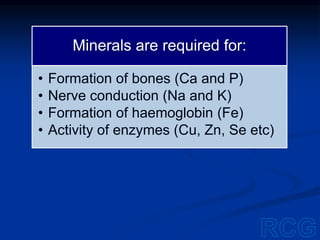
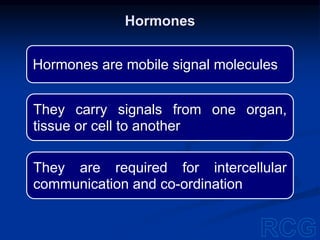
2. Within cells, these biomolecules and inorganic elements confer the property of life and allow the cell to obtain materials, produce enzymes, and undergo chemical reactions to sustain itself.
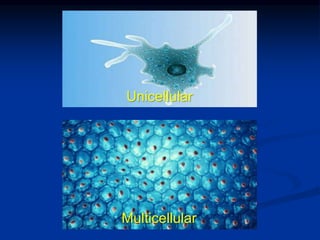
3. Biochemistry studies the biomolecules, elements, and chemical reactions within living things to understand functions at the molecular level, from single-celled to multicellular organisms.