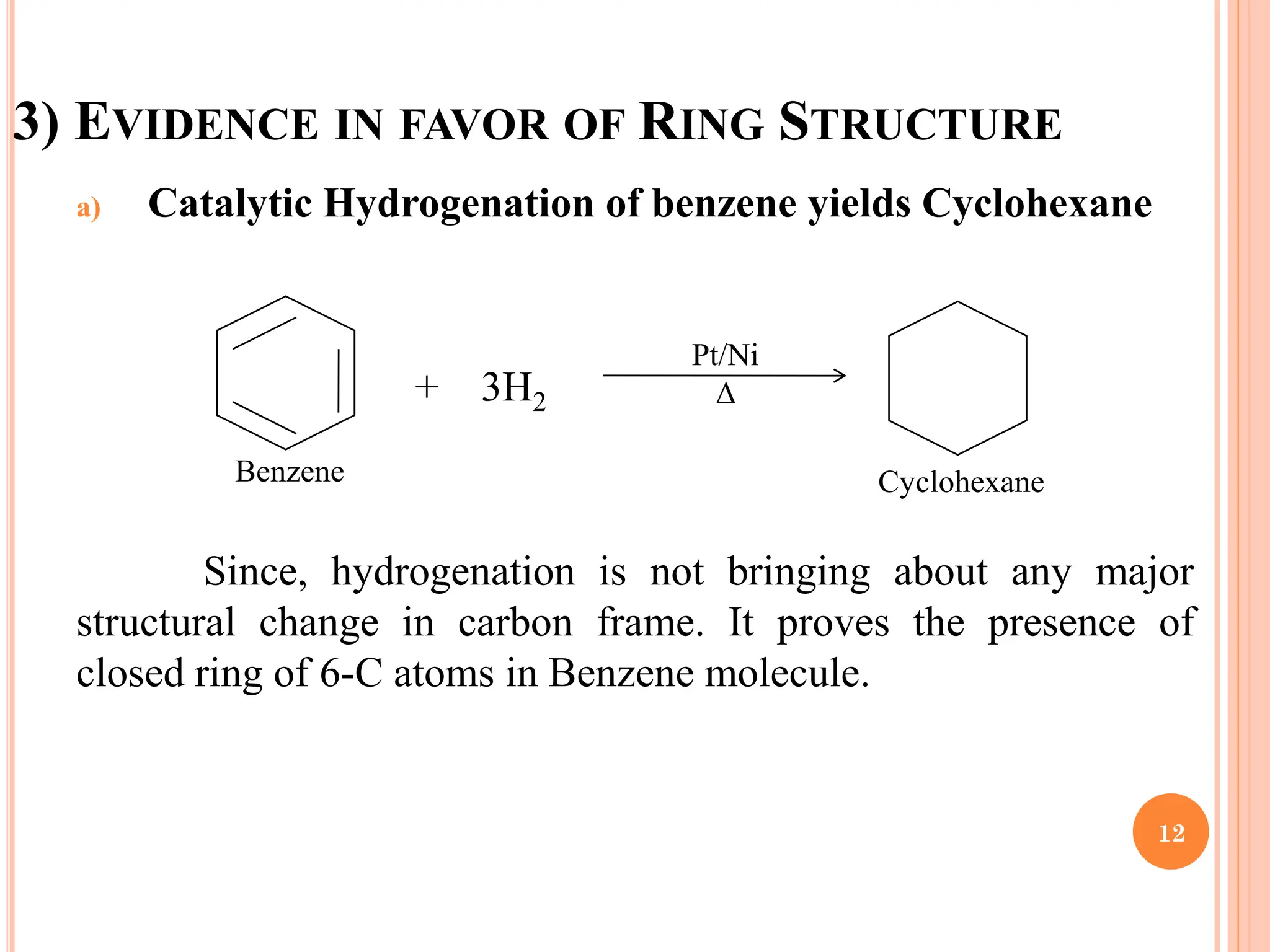
The document provides a comprehensive overview of benzene, including its physical properties, structure, nomenclature, and reactions. Benzene is a colorless, highly flammable liquid with the chemical formula C6H6, composed of six carbon and six hydrogen atoms arranged in a ring. Key topics include the evidence supporting benzene's ring structure, aromaticity, electrophilic substitution reactions, and specific reaction mechanisms such as nitration, sulfonation, and Friedel-Crafts reactions.