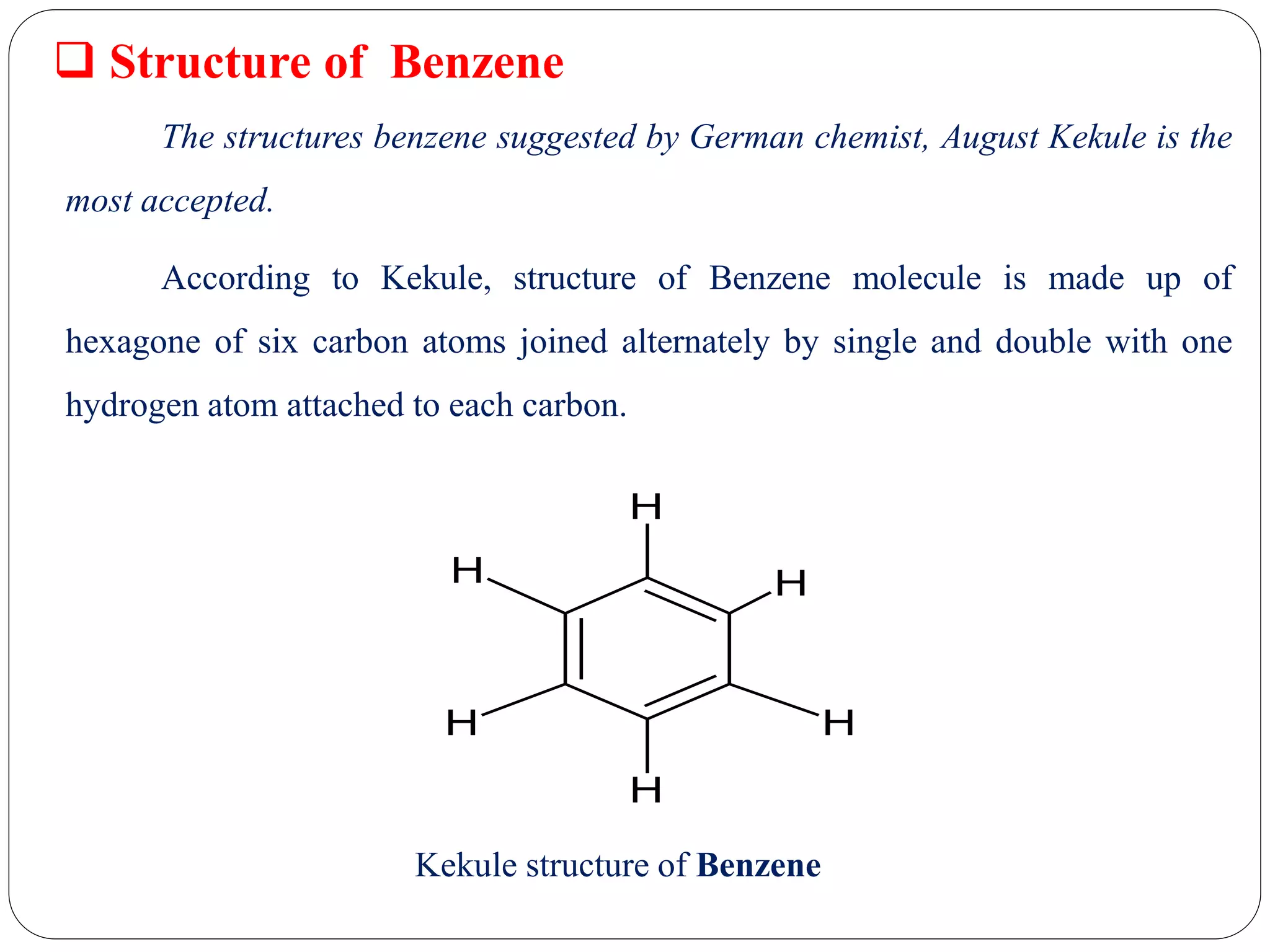
1) The document discusses the structure of benzene as proposed by German chemist August Kekule. Kekule suggested benzene's structure is a hexagonal ring of six carbon atoms with alternating single and double bonds and a hydrogen atom attached to each carbon.
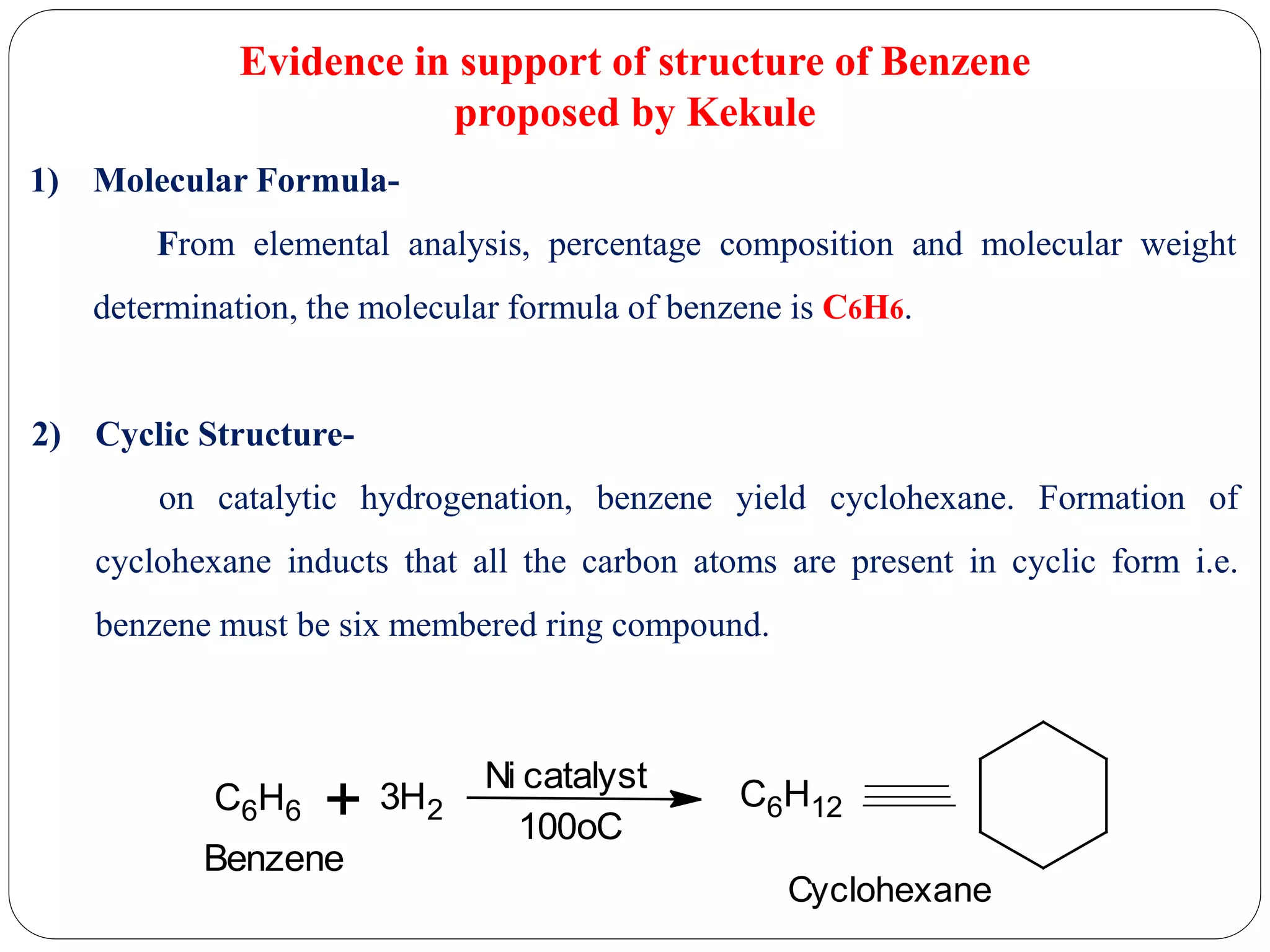
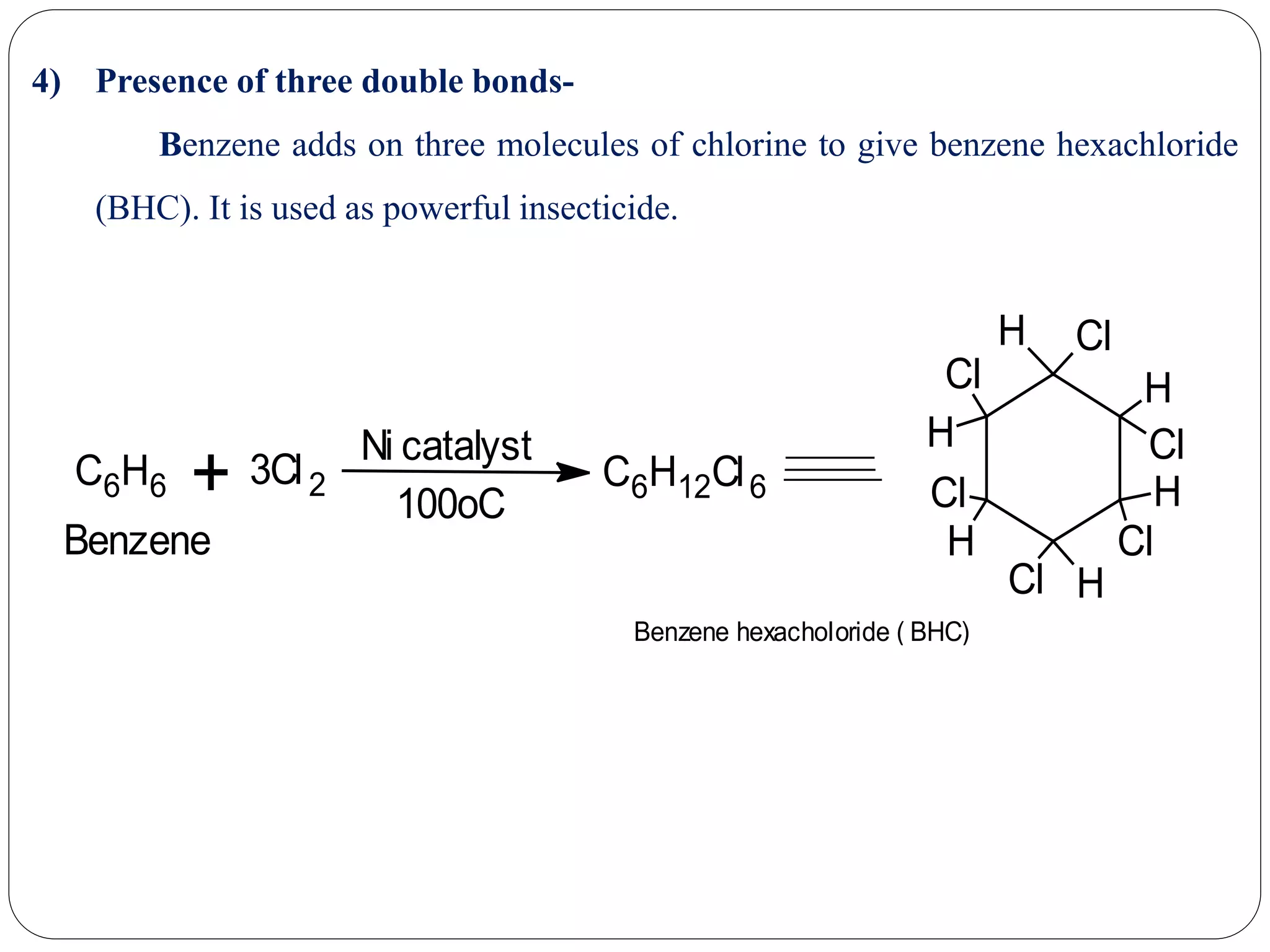
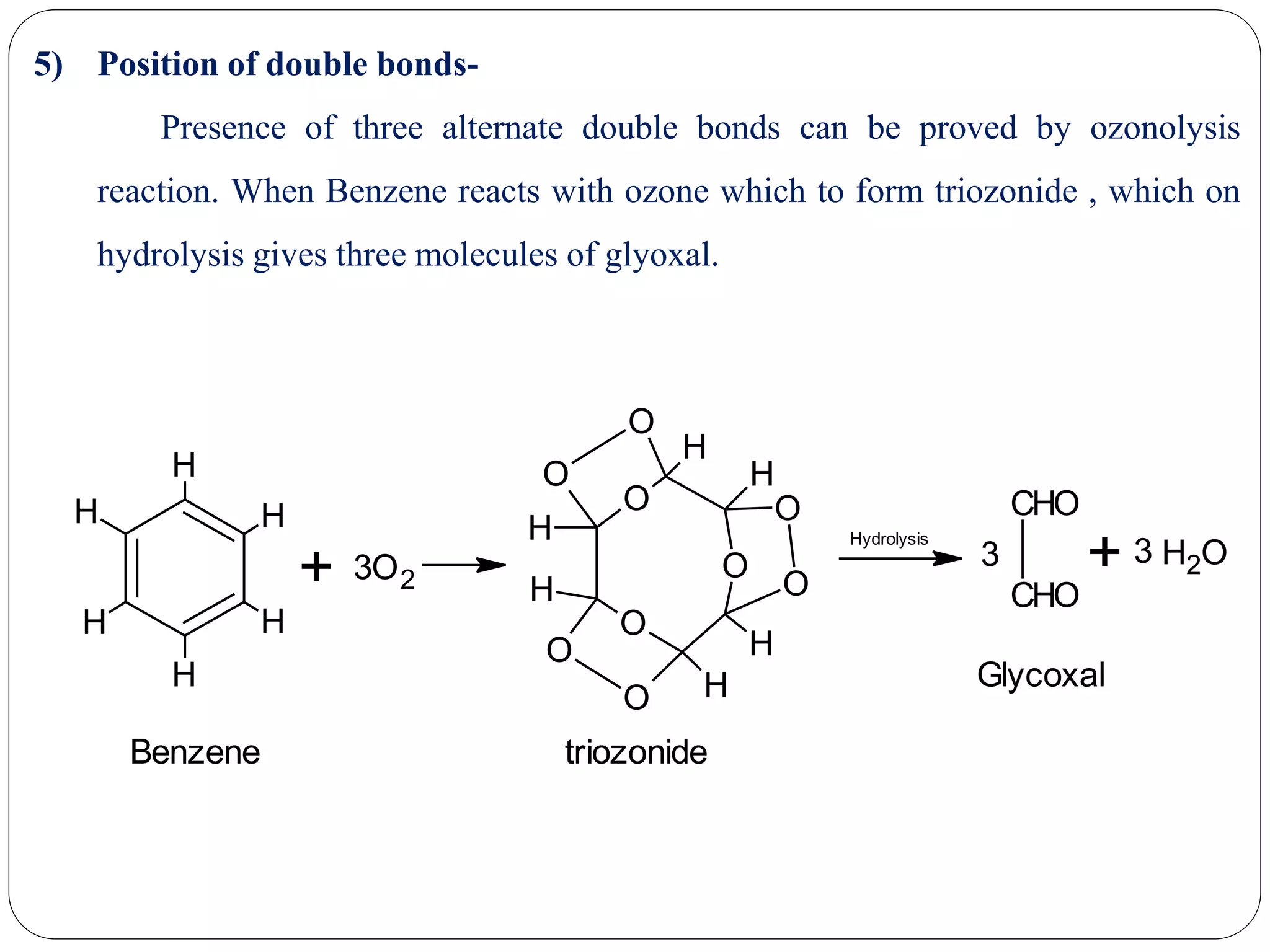
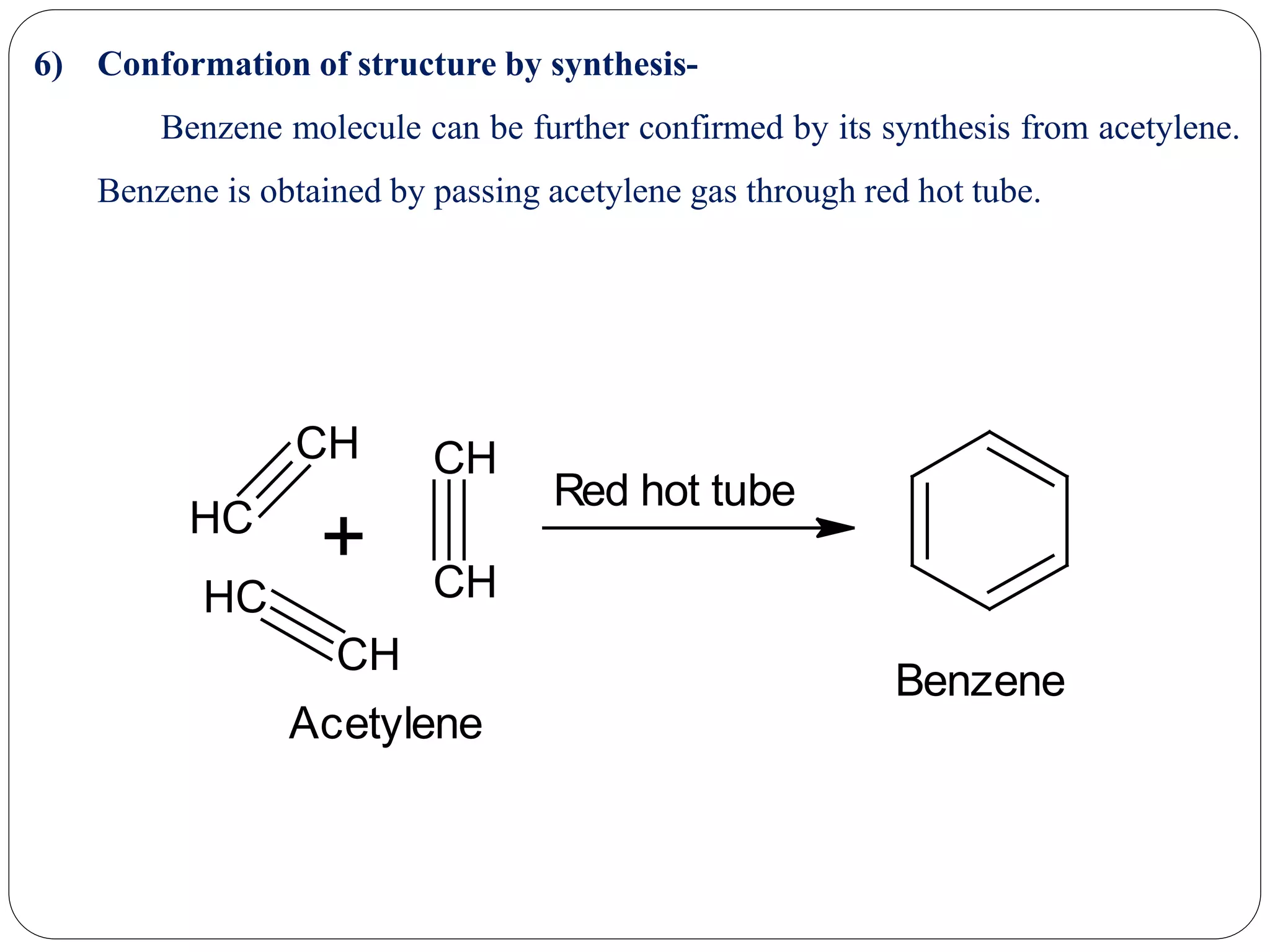
2) Evidence that supports Kekule's structure includes benzene's molecular formula of C6H6, its ability to yield cyclohexane upon hydrogenation indicating a cyclic structure, and its substitution patterns.
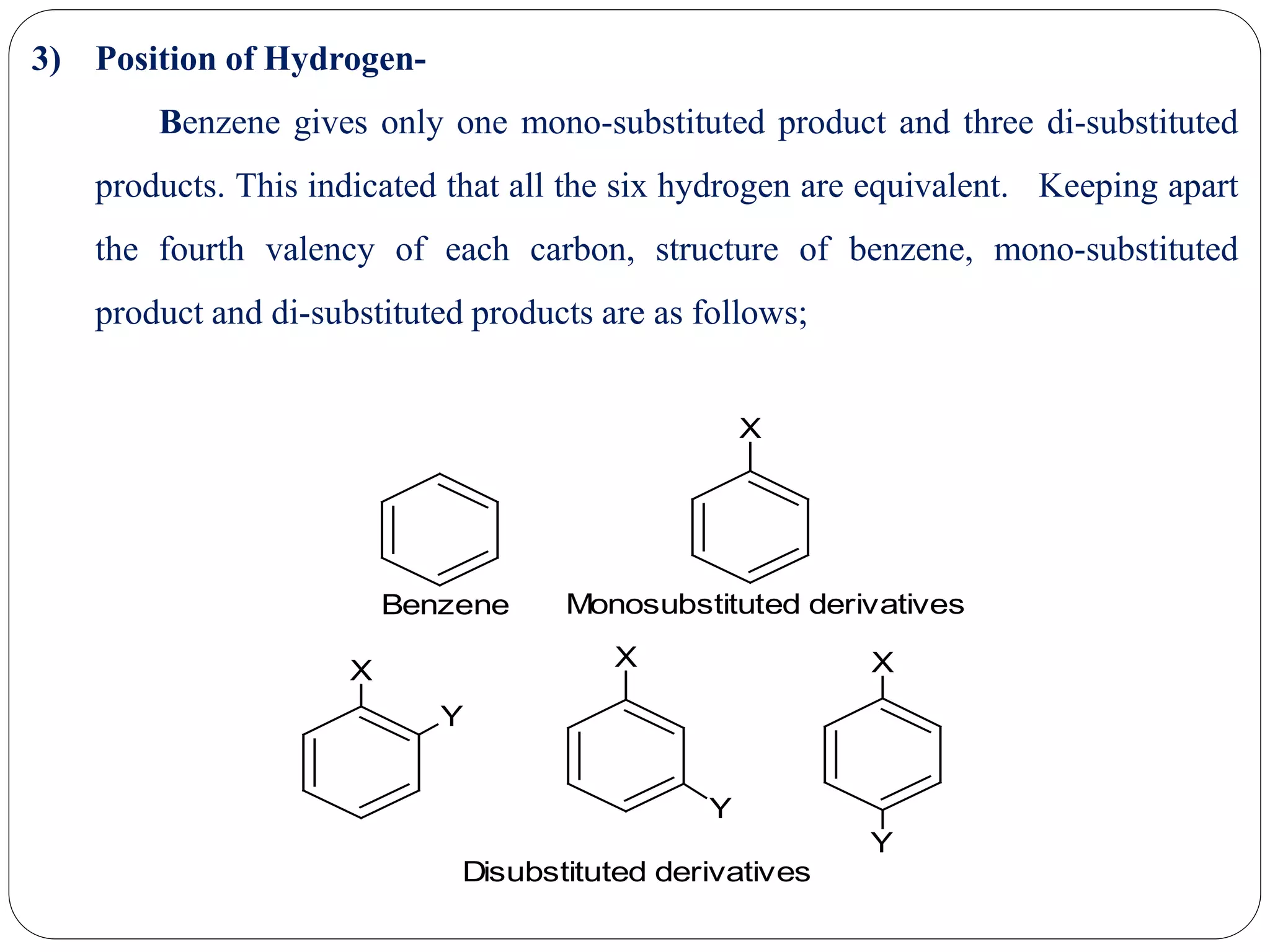
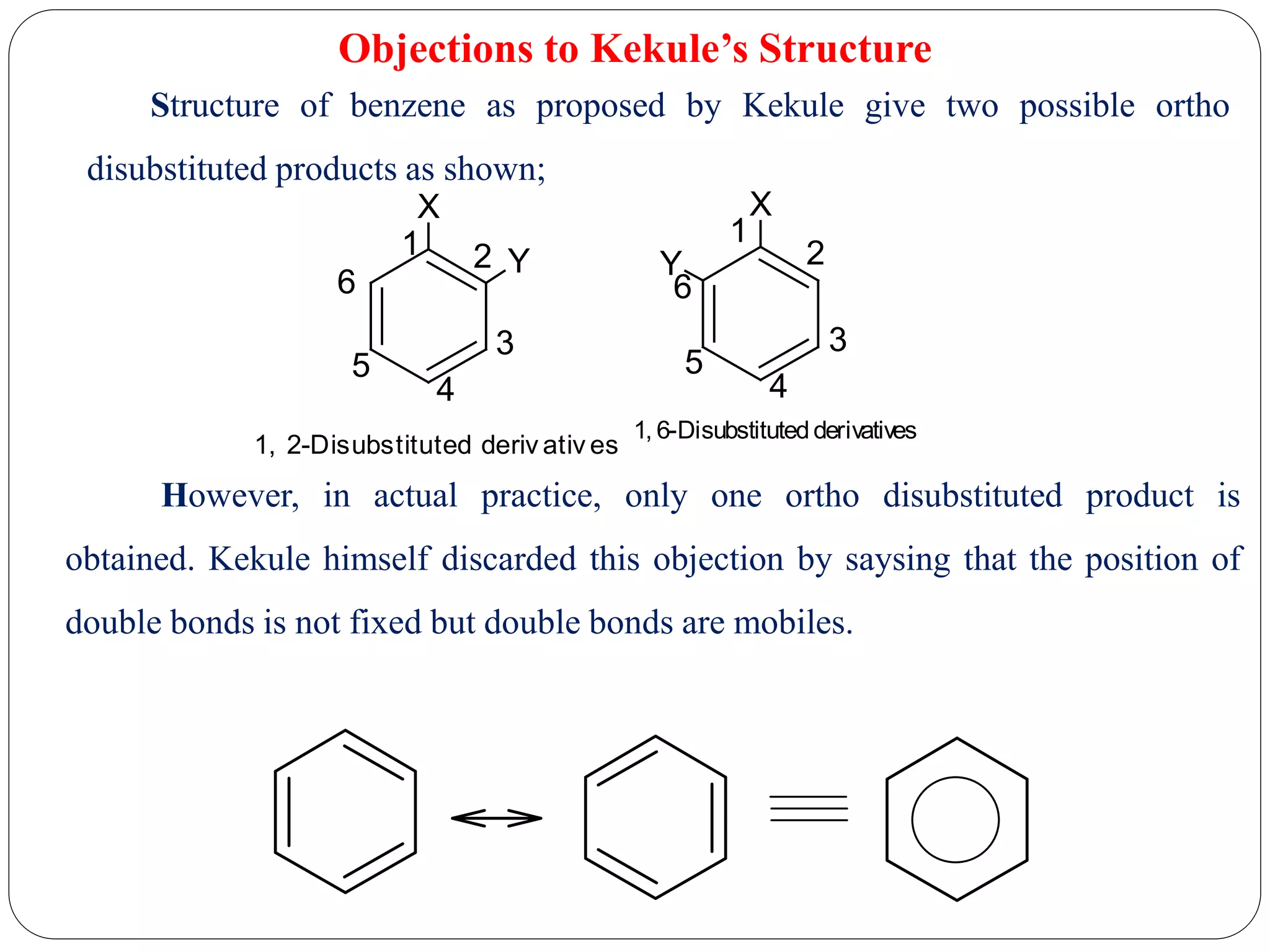
3) Objections to Kekule's structure centered around it allowing for two possible ortho disubstituted products, but in practice only one is observed. Kekule addressed this by proposing the double bonds are mobile within the