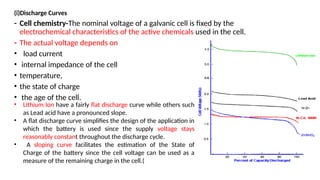
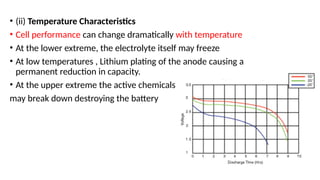
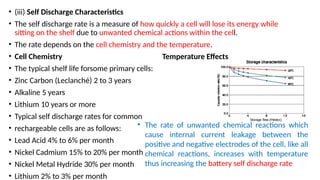
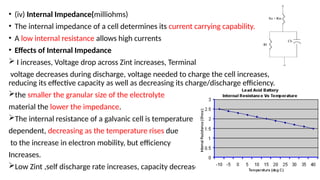
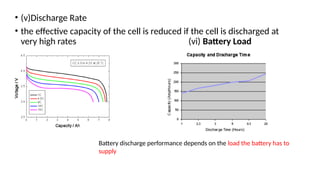
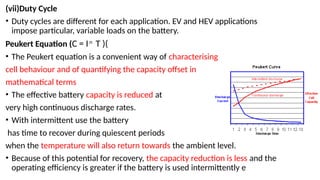
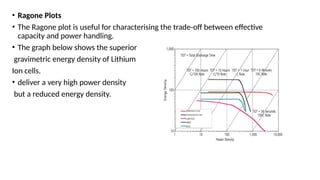
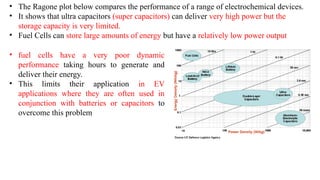
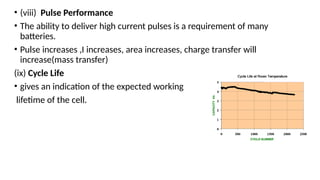
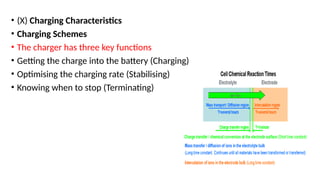
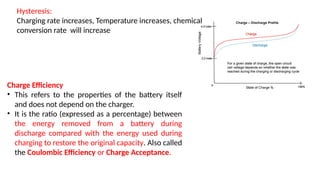
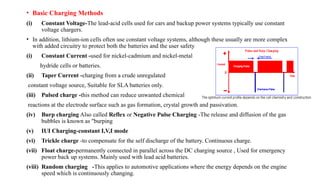
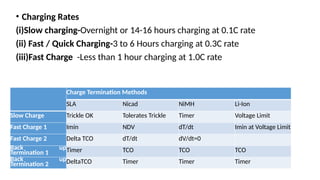
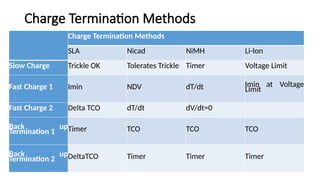
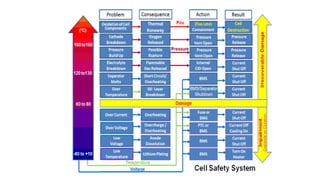
The document outlines various international battery standards and safety regulations, including those from IEC, ANSI, and ISO related to battery design, performance, and safety. It discusses battery performance characteristics such as discharge curves, temperature effects, self-discharge rates, and charging methods, aiming to provide guidelines for optimal usage and safety. Additionally, it emphasizes the importance of safety measures throughout the battery's lifecycle, from design to end-user use, to prevent hazards associated with battery malfunctions.