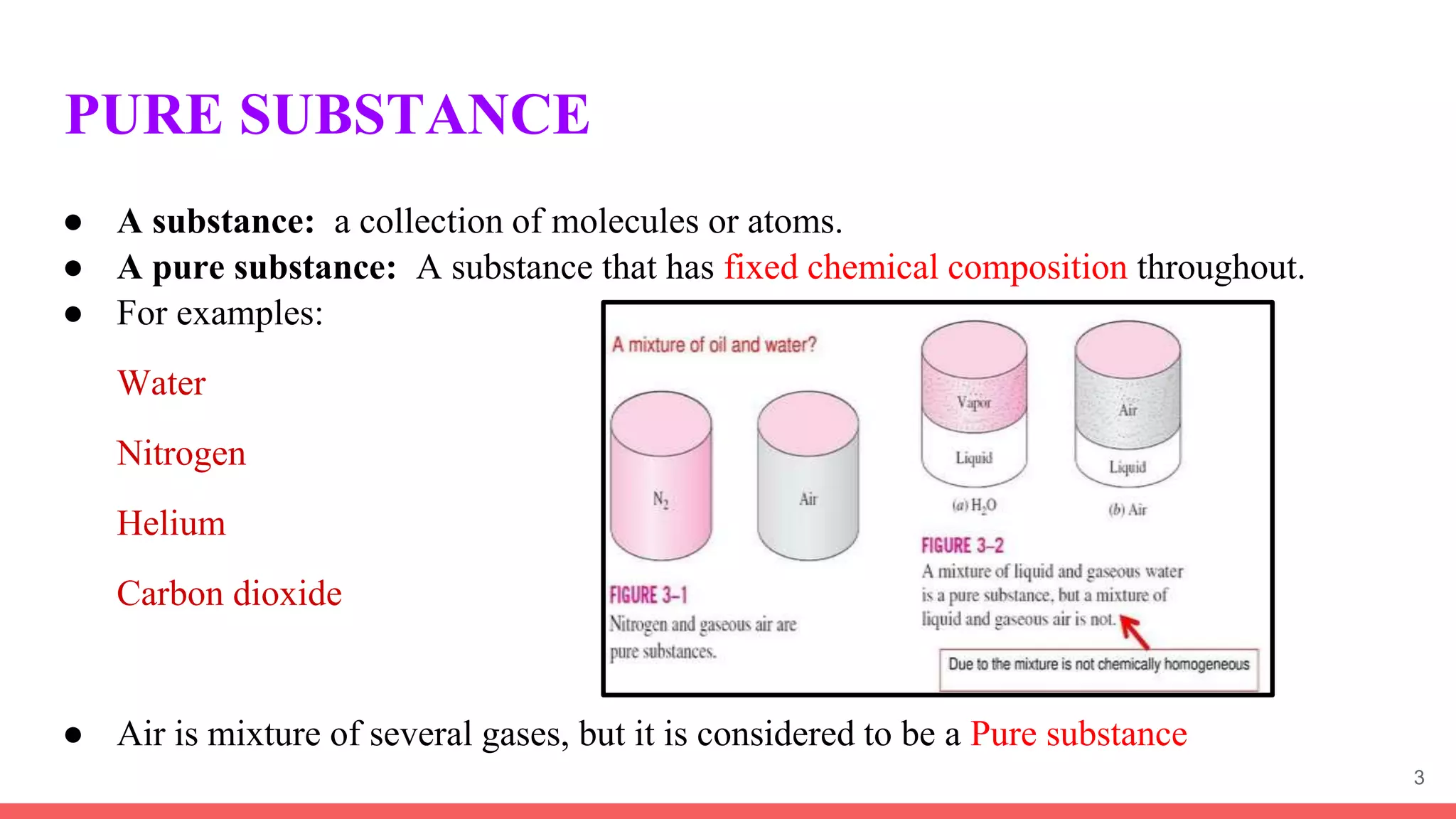
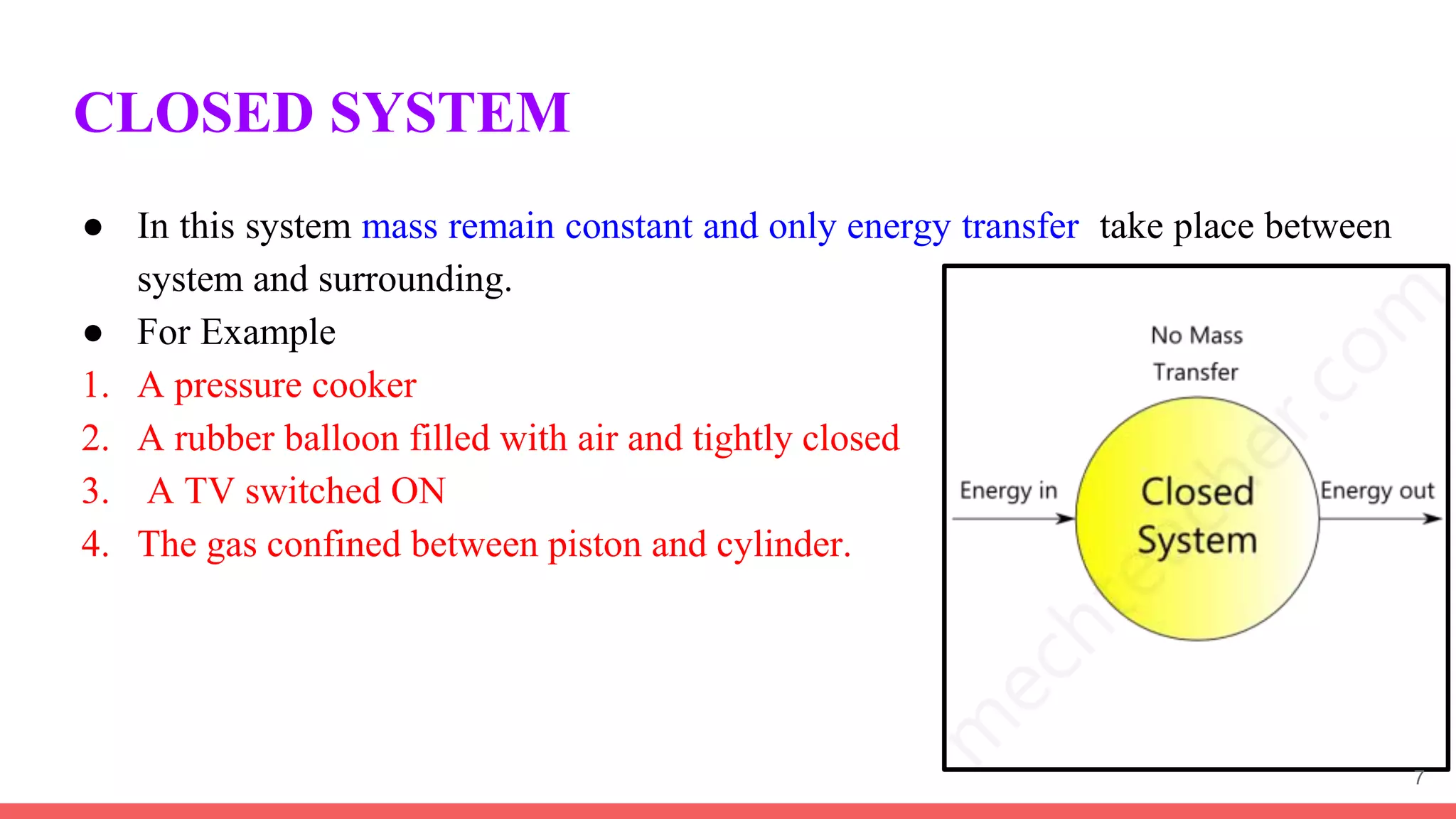
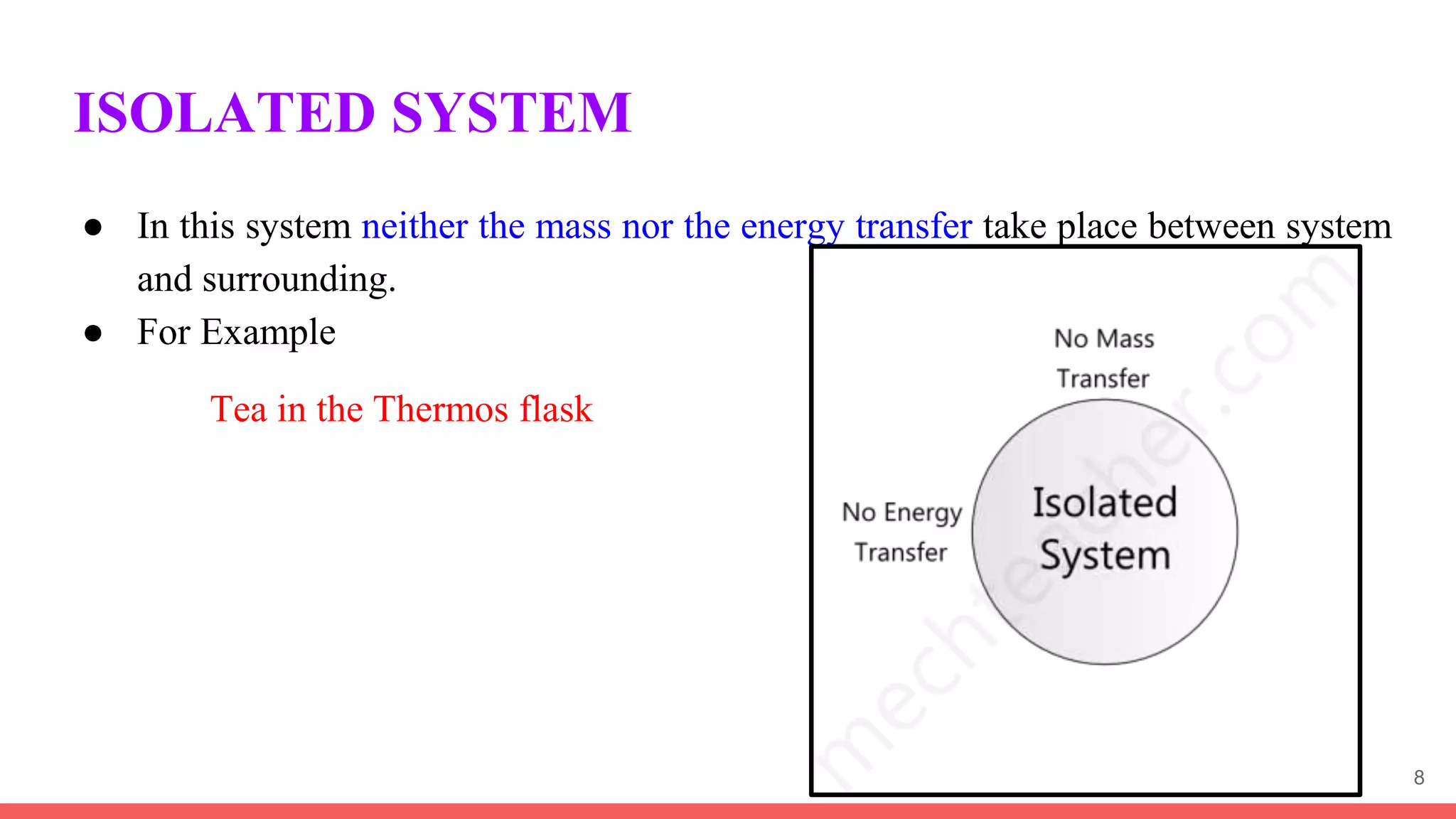
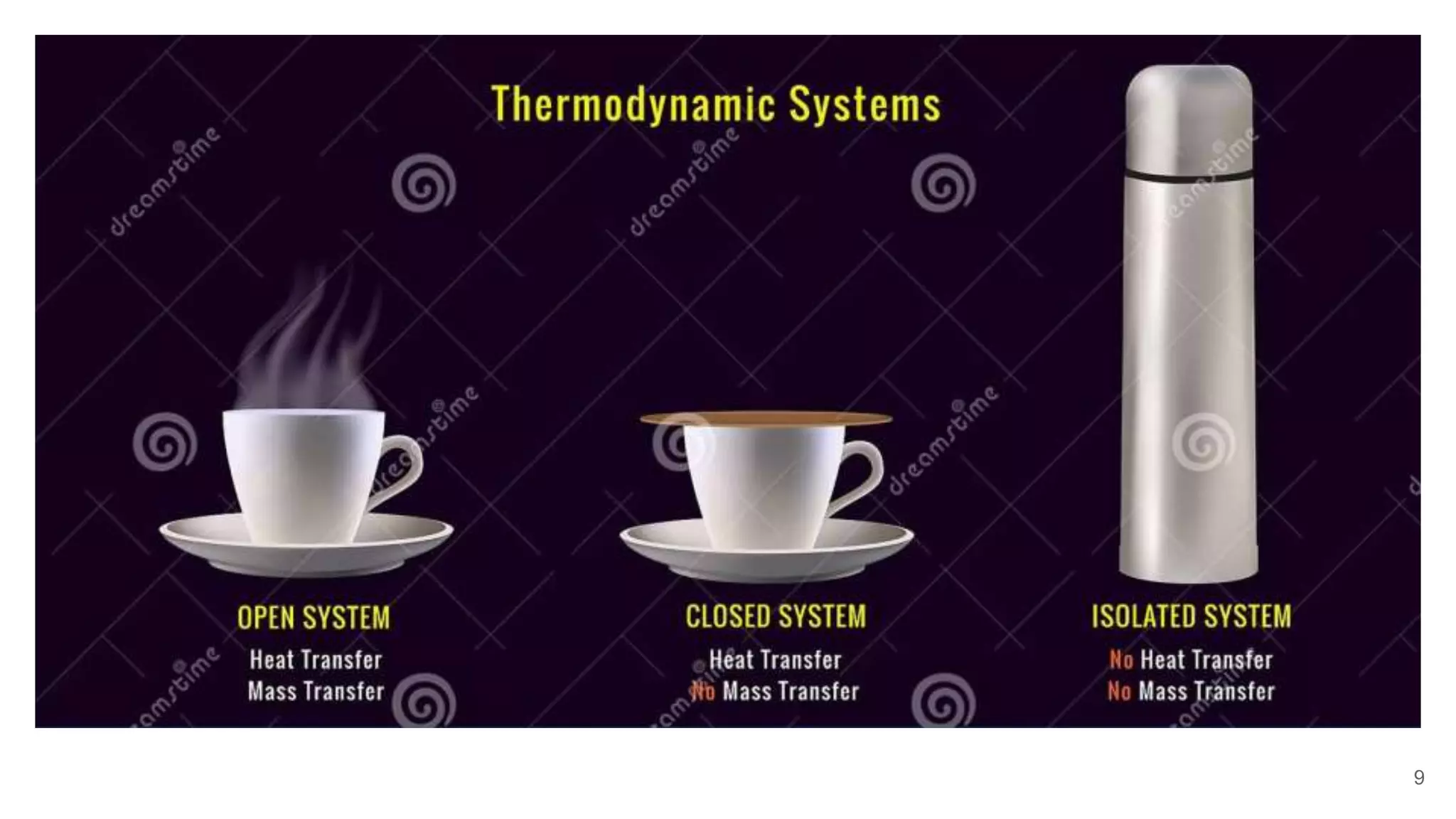
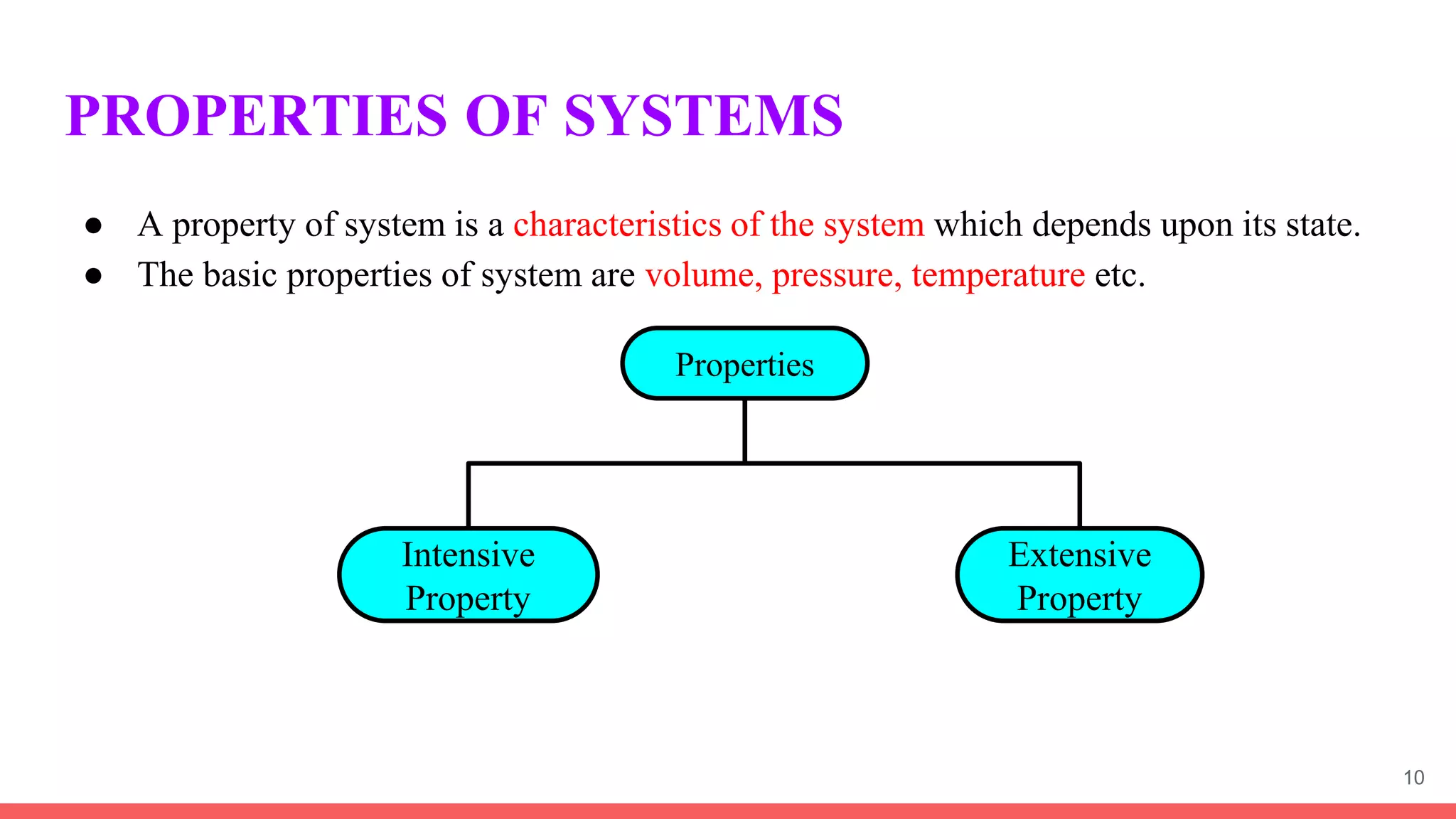
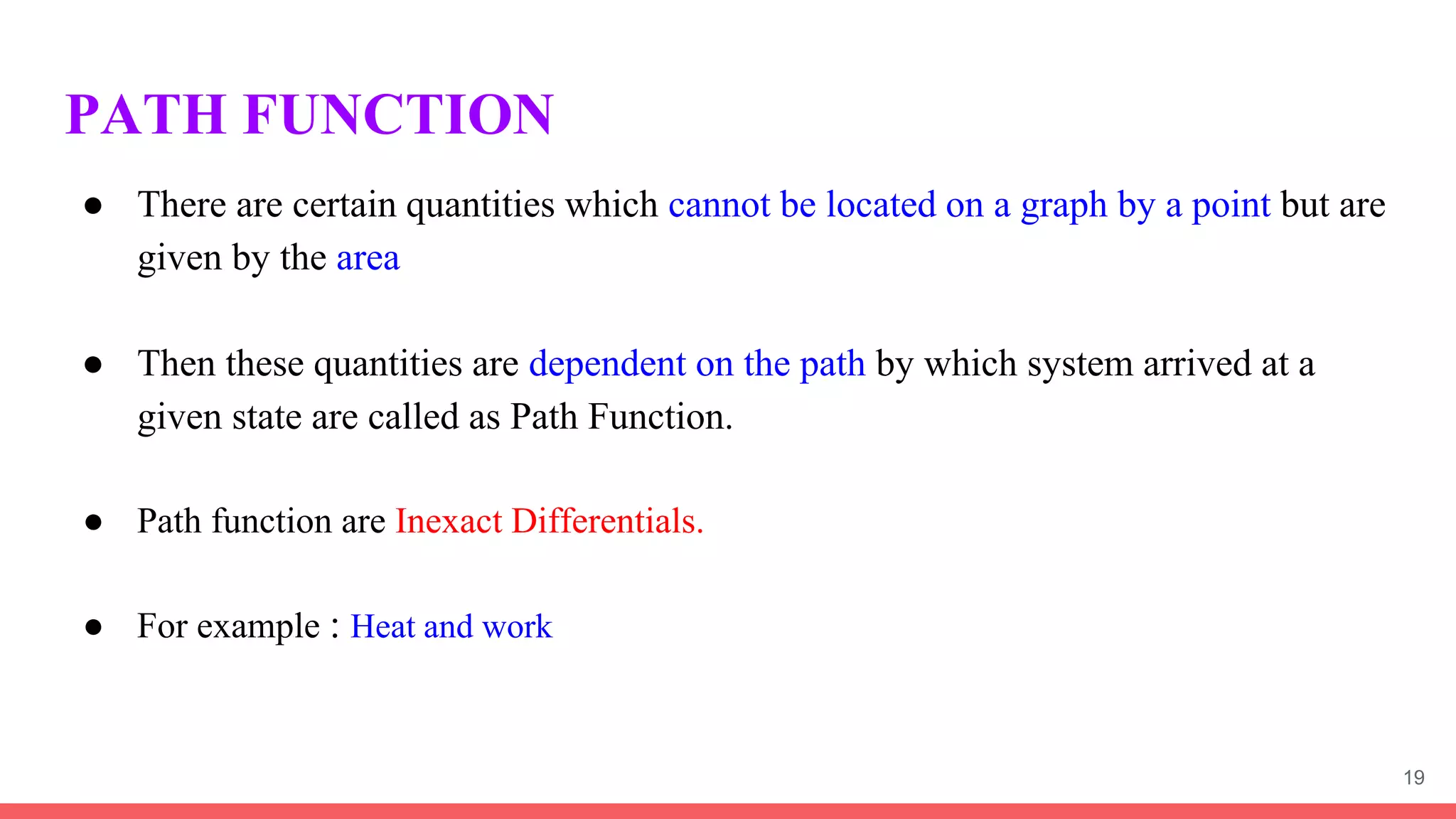
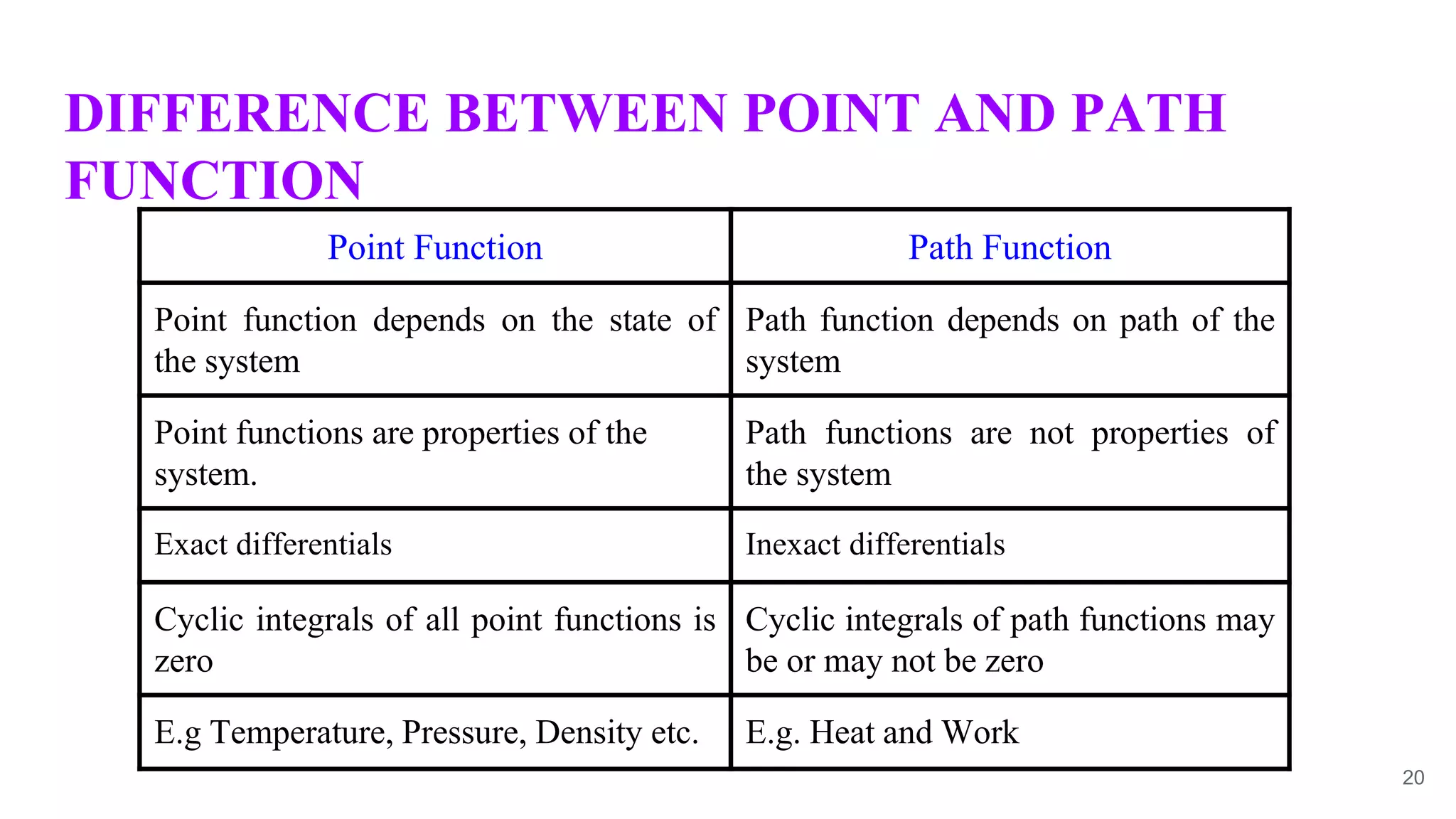
Thermodynamics is the science that deals with interactions between energy and heat. It entails four main laws. A thermodynamic system is anything considered for analysis, with boundaries separating it from its surroundings. Systems can be open, closed, or isolated depending on mass and energy transfer. Thermodynamic properties describe a system's state and can be intensive or extensive. A thermodynamic process occurs as a system changes state, and can involve flow or no flow of mass. Basic thermodynamic properties include volume, temperature, pressure, specific volume, and density.