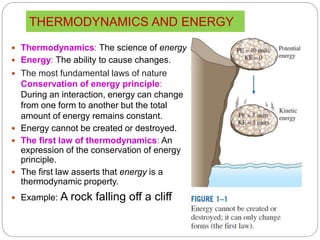
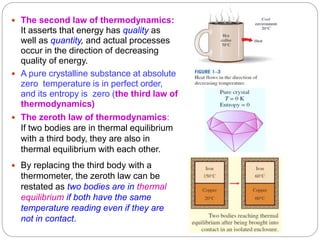
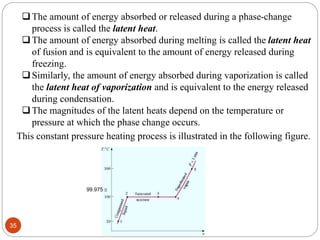
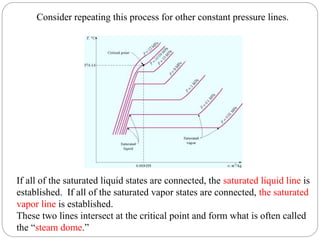
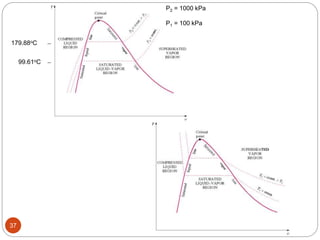
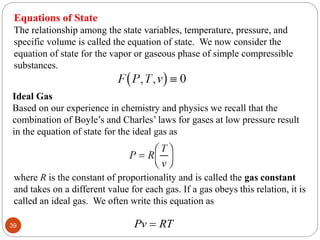
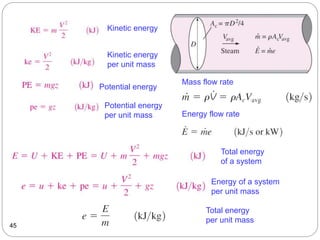
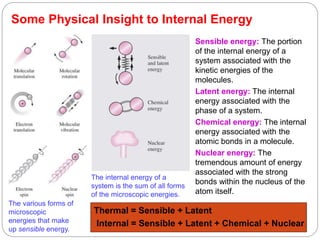
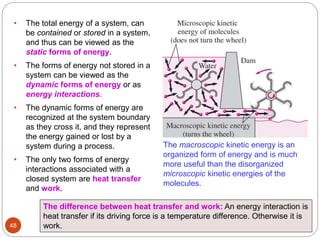
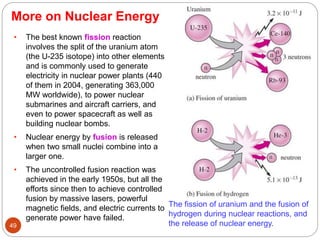
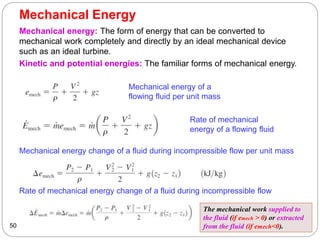
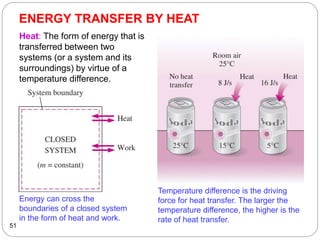
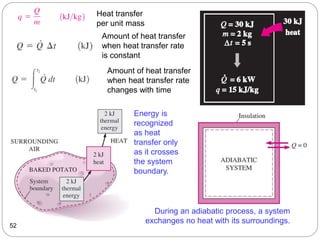
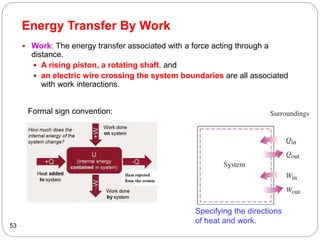
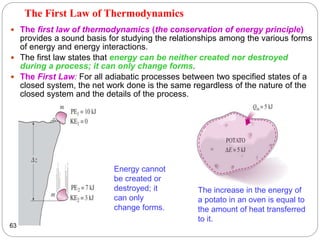
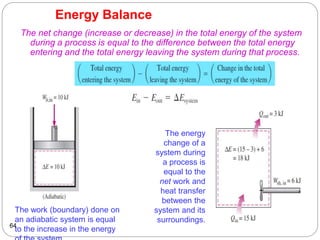
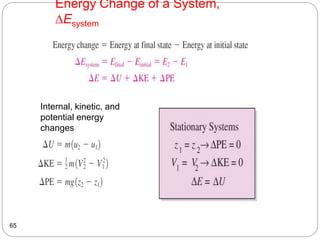
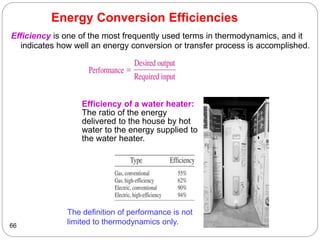
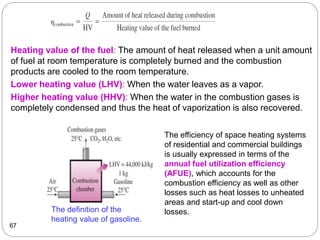
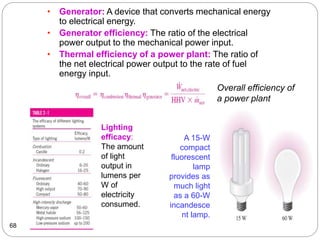
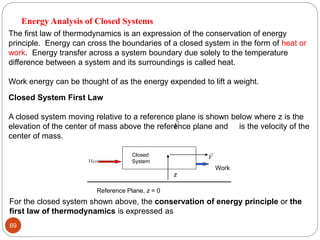
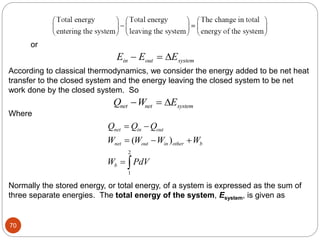
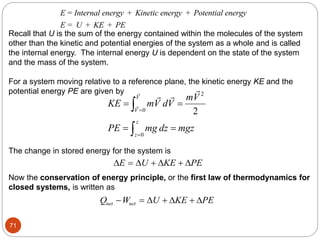
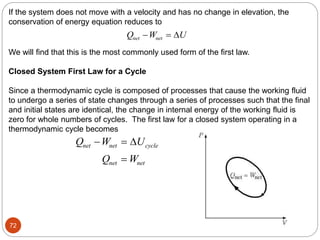
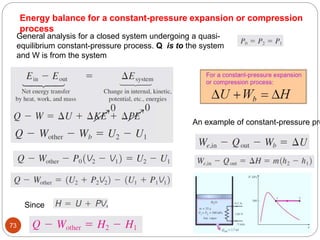
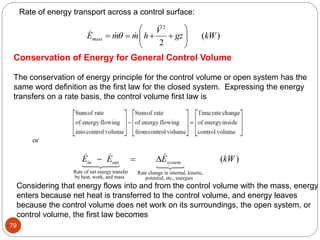
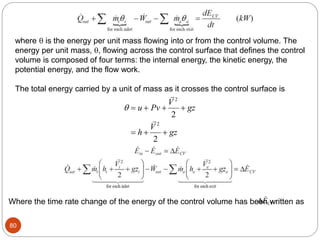
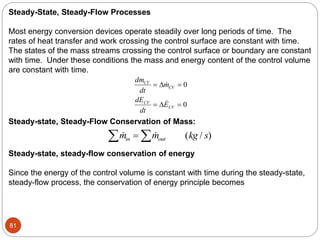
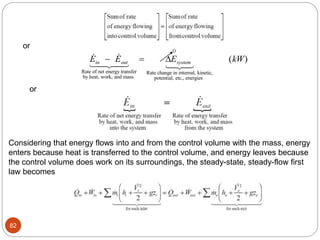
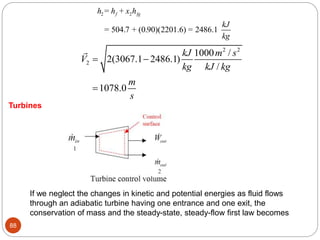
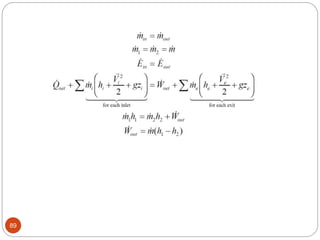
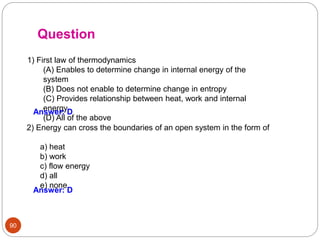
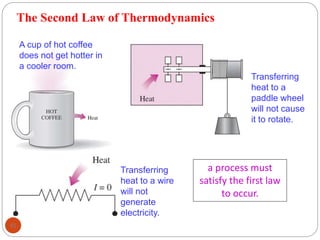
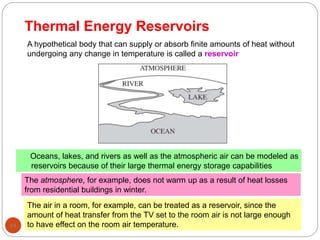
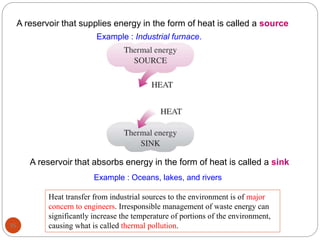
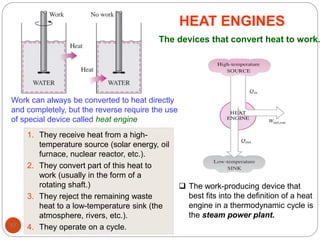
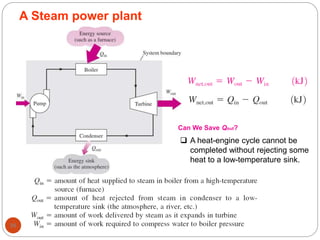
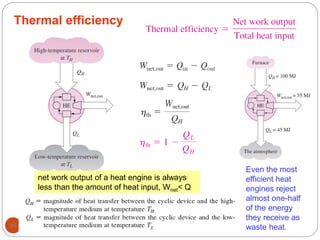
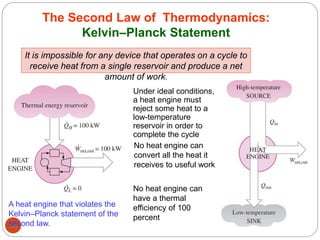
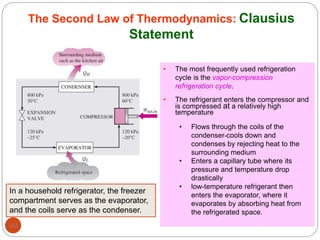
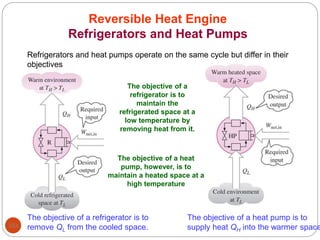
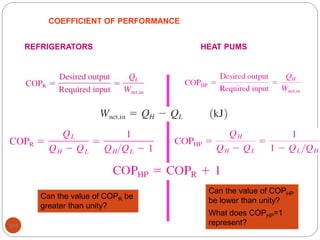
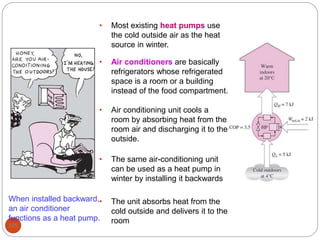
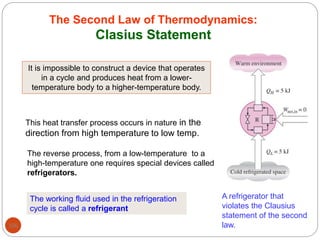
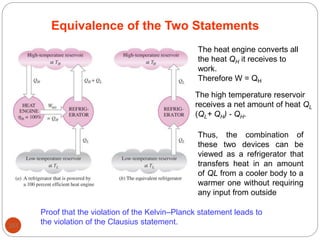
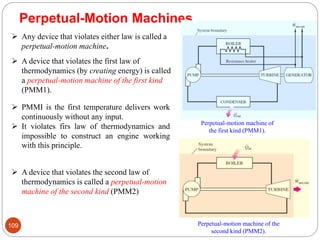
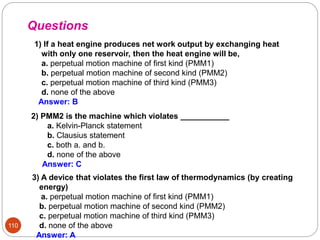
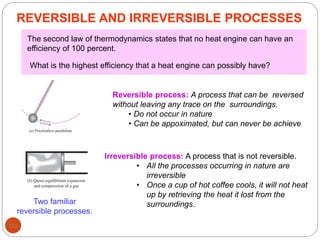
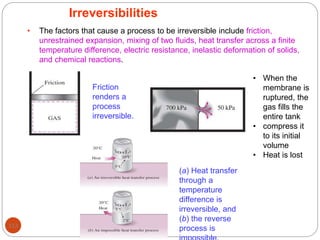
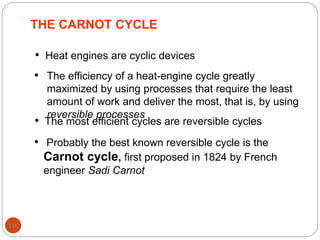
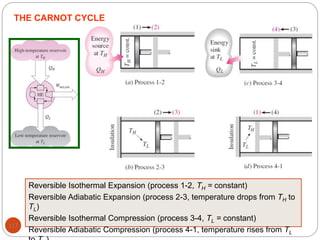
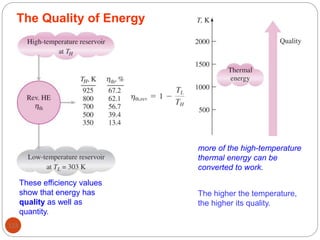
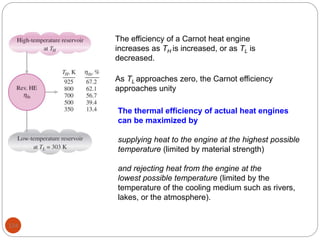
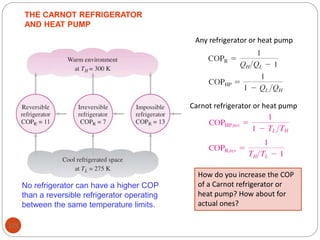
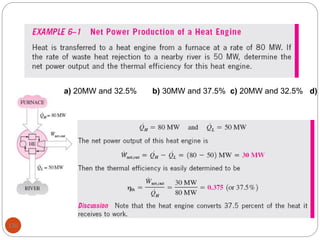
This document provides an overview of thermodynamics concepts including:
- The four laws of thermodynamics and what they assert about energy and its transformation.
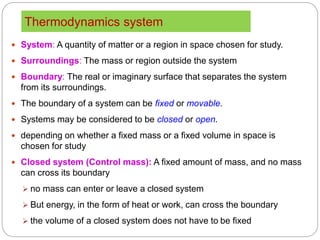
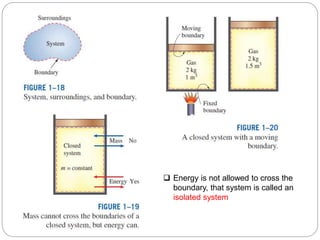
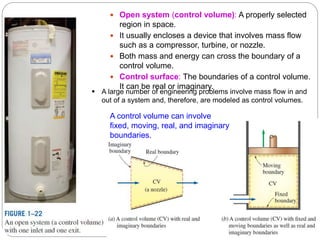
- Systems, surroundings, boundaries, open and closed systems.
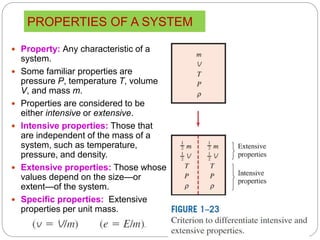
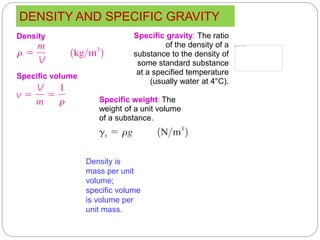
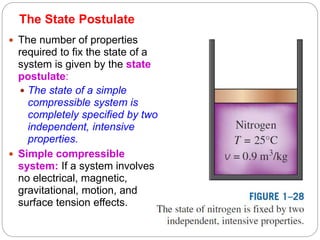
- Properties of systems including intensive, extensive, specific properties, and state properties.
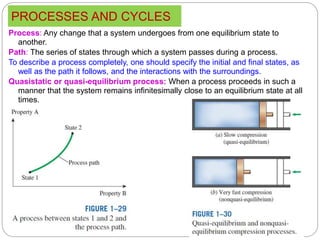
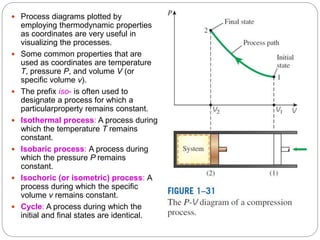
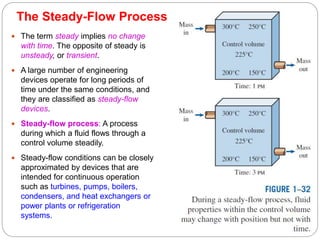
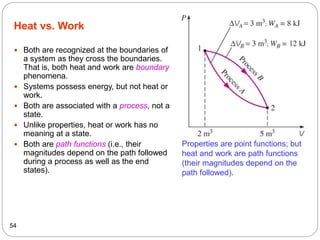
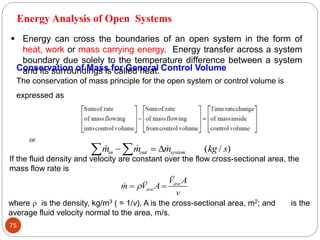
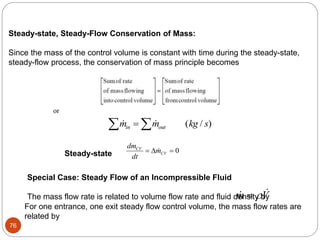
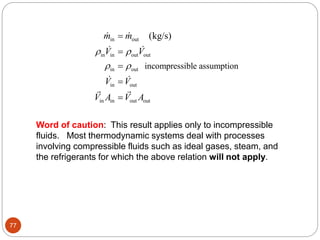
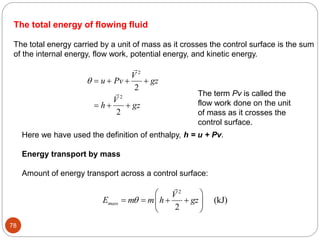
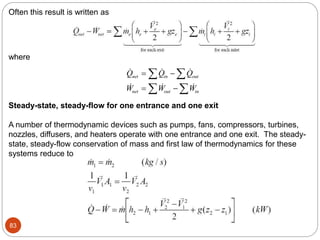
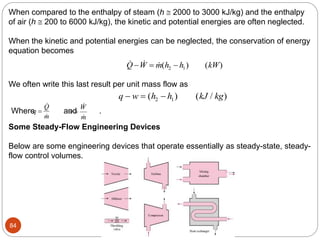
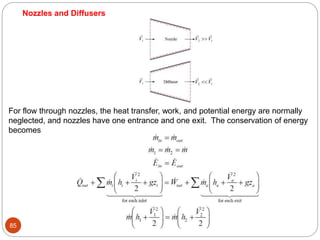
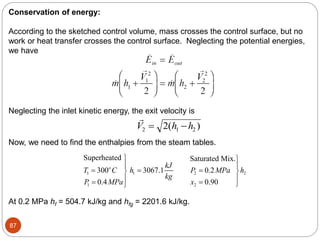
- Equilibrium states, processes, cycles, and steady-flow processes.
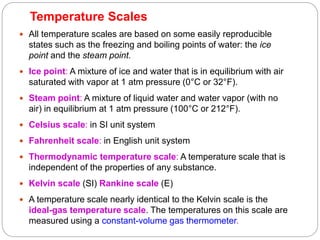
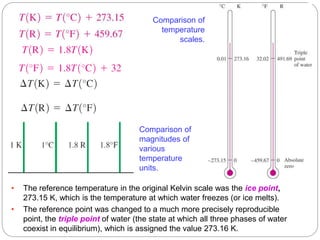
- Temperature scales including Celsius, Fahrenheit, Kelvin, and Rankine scales.
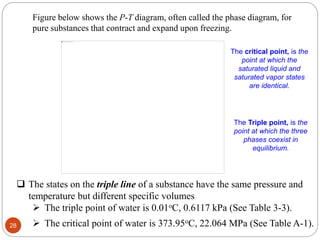
- Concepts related to pure substances including homogeneous substances, phases, and examples.