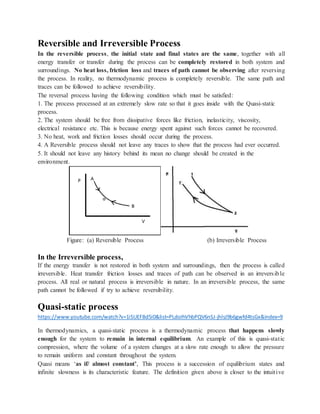
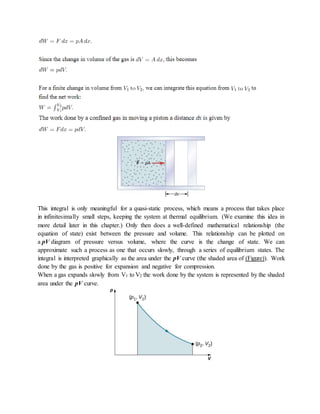
This document provides notes on thermodynamics for a mechanical engineering course, covering fundamental concepts, definitions, and classifications of thermodynamic systems. It includes discussions on energy conversion, properties of substances, equilibrium states, and the distinctions between various types of processes such as reversible and irreversible. Additionally, the document emphasizes the importance of thermodynamic principles in applications such as power generation and automotive engineering.