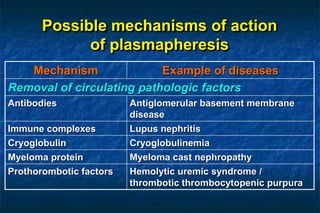
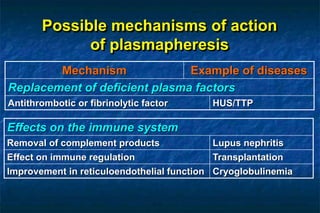
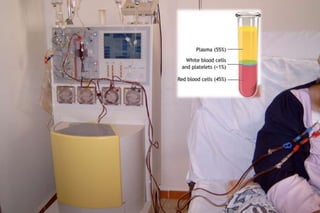
1. Plasmapheresis involves removing plasma from a patient and replacing it with either fresh frozen or stored plasma. It can remove pathogenic factors like antibodies, immune complexes, and proteins.
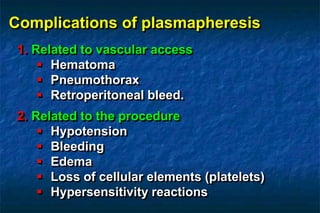
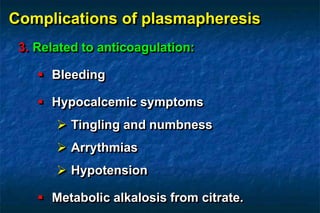
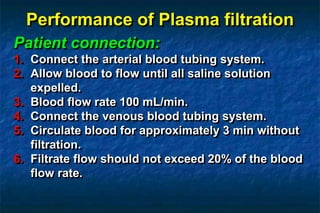
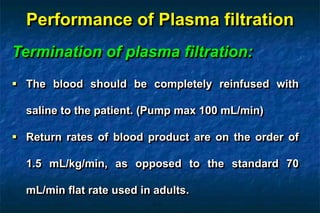
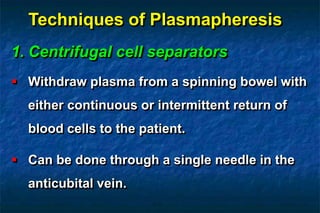
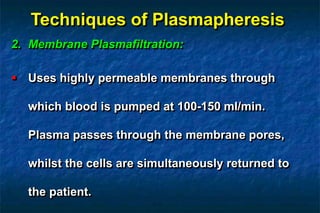
2. There are two main techniques for plasmapheresis - centrifugal separation and membrane plasmafiltration. Complications can include hypotension, bleeding, and allergic reactions.
3. Plasmapheresis is used to treat various conditions and is categorized based on evidence. It may be used as an adjunctive therapy for sepsis to remove harmful molecules.












![Estimation of plasma volume
35-40 mL/kg body weight
Kaplan's equation
[0.065 x weight (kg)] x (1- Hct)](https://image.slidesharecdn.com/basicplasmapheresisprof-171030152449/85/Basic-plasmapheresis-prof-dr-montasser-zeid-19-320.jpg)









![Category IV (disorders in which published evidence
demonstrates or suggests apheresis to be ineffective or
harmful; institutional review board [IRB] approval is
desirable if apheresis treatment is undertaken in these
circumstances) are as follows:
Stiff person syndrome
Hemolytic uremic syndrome (typical diarrhea-associated)
Systemic lupus erythematosus (nephritis)
Immune thrombocytopenia](https://image.slidesharecdn.com/basicplasmapheresisprof-171030152449/85/Basic-plasmapheresis-prof-dr-montasser-zeid-29-320.jpg)