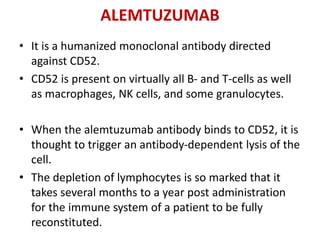
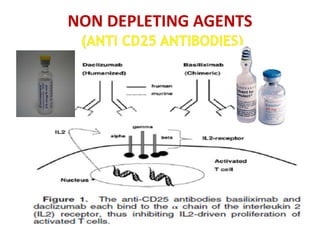
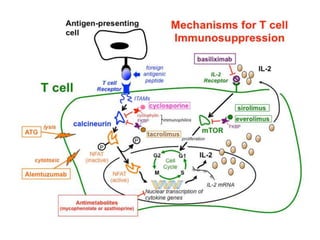
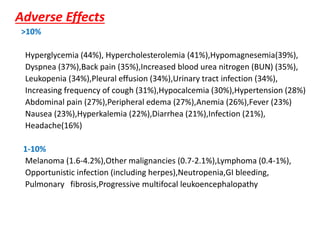
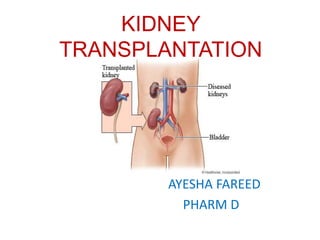
Kidney transplantation involves the surgical implantation of a healthy kidney into a patient with end-stage renal disease and can involve living or deceased donors. Successful transplantation relies on proper donor-recipient matching, which includes blood type and tissue typing, as well as management of potential rejection, which can occur in different forms post-surgery. Immunosuppressive therapies, including induction and maintenance therapies, are critical for preventing rejection and ensuring the success of the transplant.




![DRUGS USED IN KIDNEY TRANSPLANT
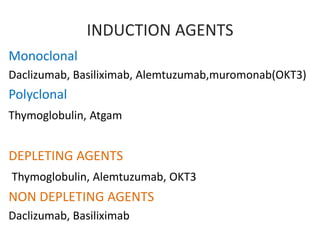
INDUCTION THERAPY
1)monoclonal antibodies:
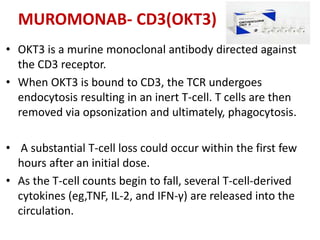
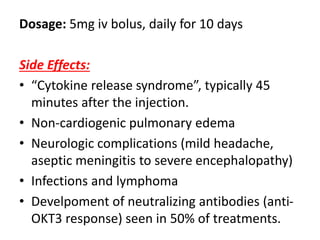
muromonab-CD3,
basiliximab
DaclizumaB
Alemtuzumab
2) polyclonal antibodies:
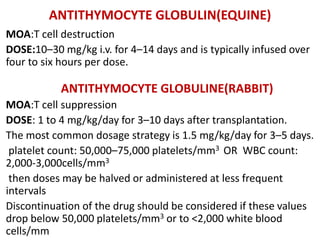
Antithymocyte globulin
[equine] Antithymocyte
globulin [rabbit])
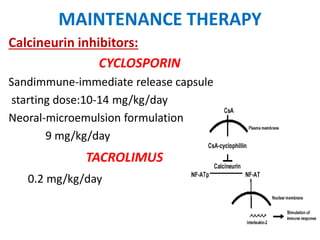
MAINTENANENCE THERAPY
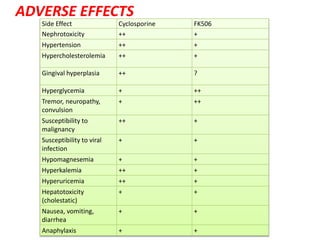
1)Calcineurin inhibitors
– Cyclosporine
– Tacrolimus
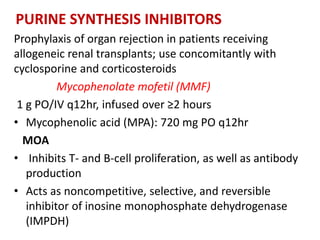
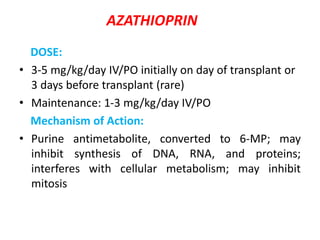
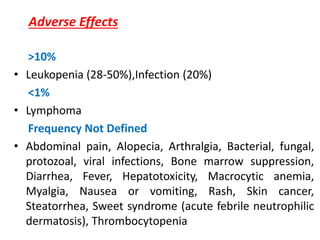
2)Purine synthesis
inhibitors/APA
– Azathioprine
– Mycophenolate mofetil
3)steroids
– prednisone
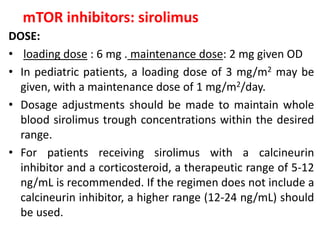
4)mTOR inhibitors
– Sirolimus](https://image.slidesharecdn.com/kt-160315162644/85/KIDNEY-TRANSPLANTATION-SEMINAR-PRESENTATION-9-320.jpg)