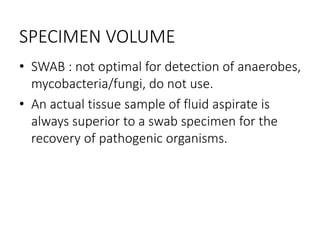
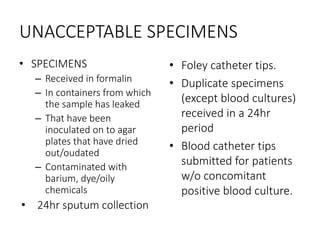
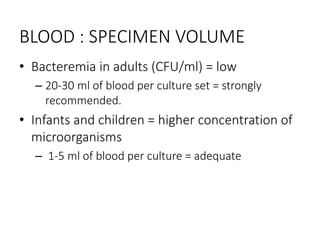
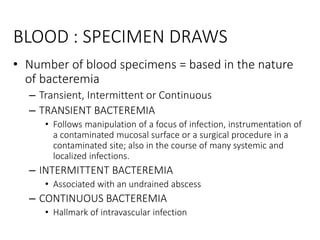
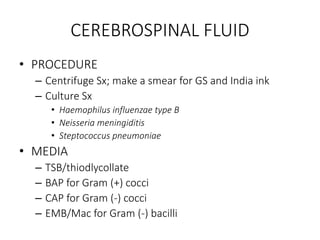
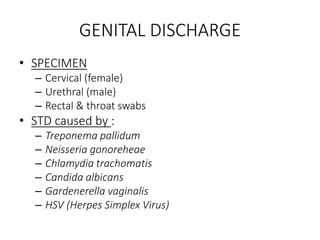
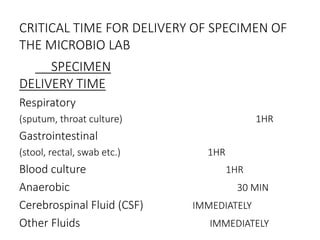
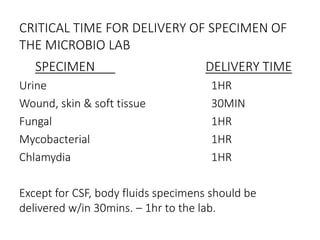
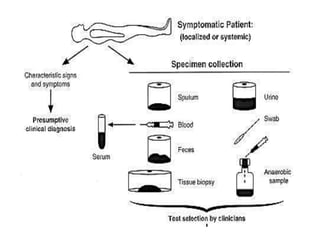
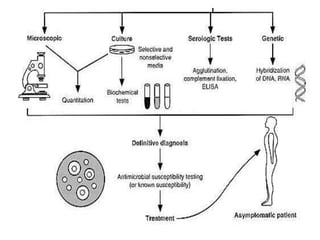
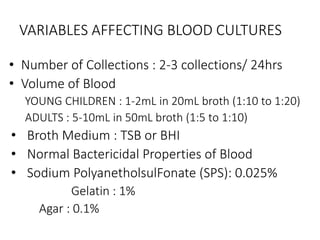
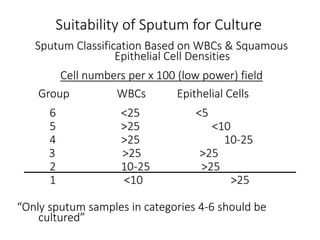
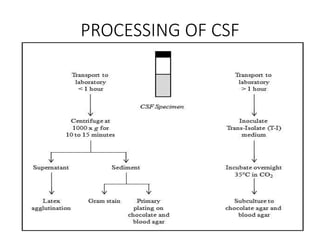
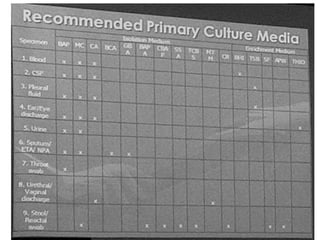
Specimen collection, processing, and handling are important for accurate microbiological testing. Key points include collecting specimens before initiating antimicrobial therapy, using adequate volumes, and expediting transport. Proper collection methods and transport media are needed to preserve specimen integrity. Timing of collection depends on the suspected pathogen. Adequate labeling and packaging is required for referral testing. Universal precautions must be followed when handling all specimens.