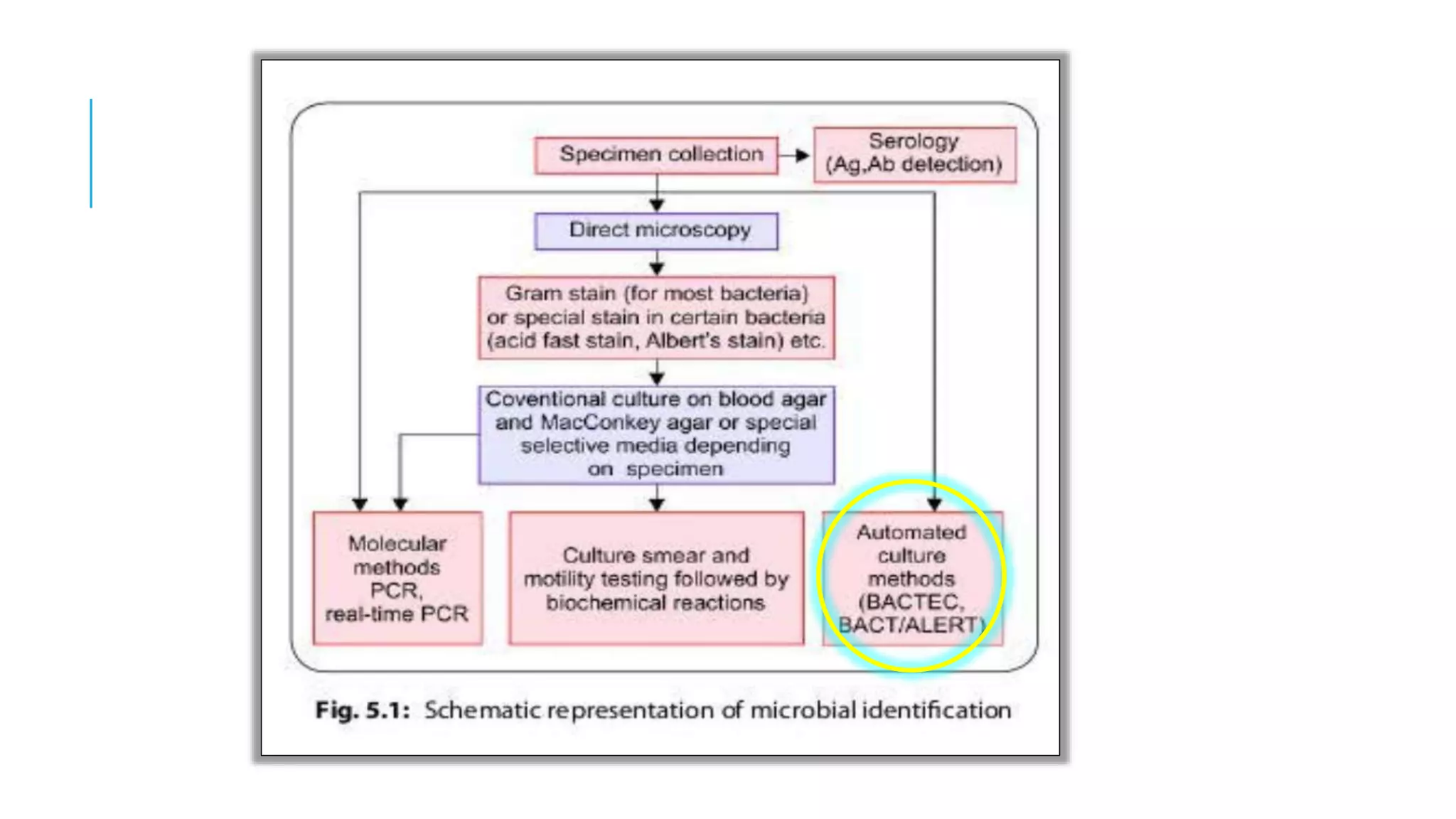
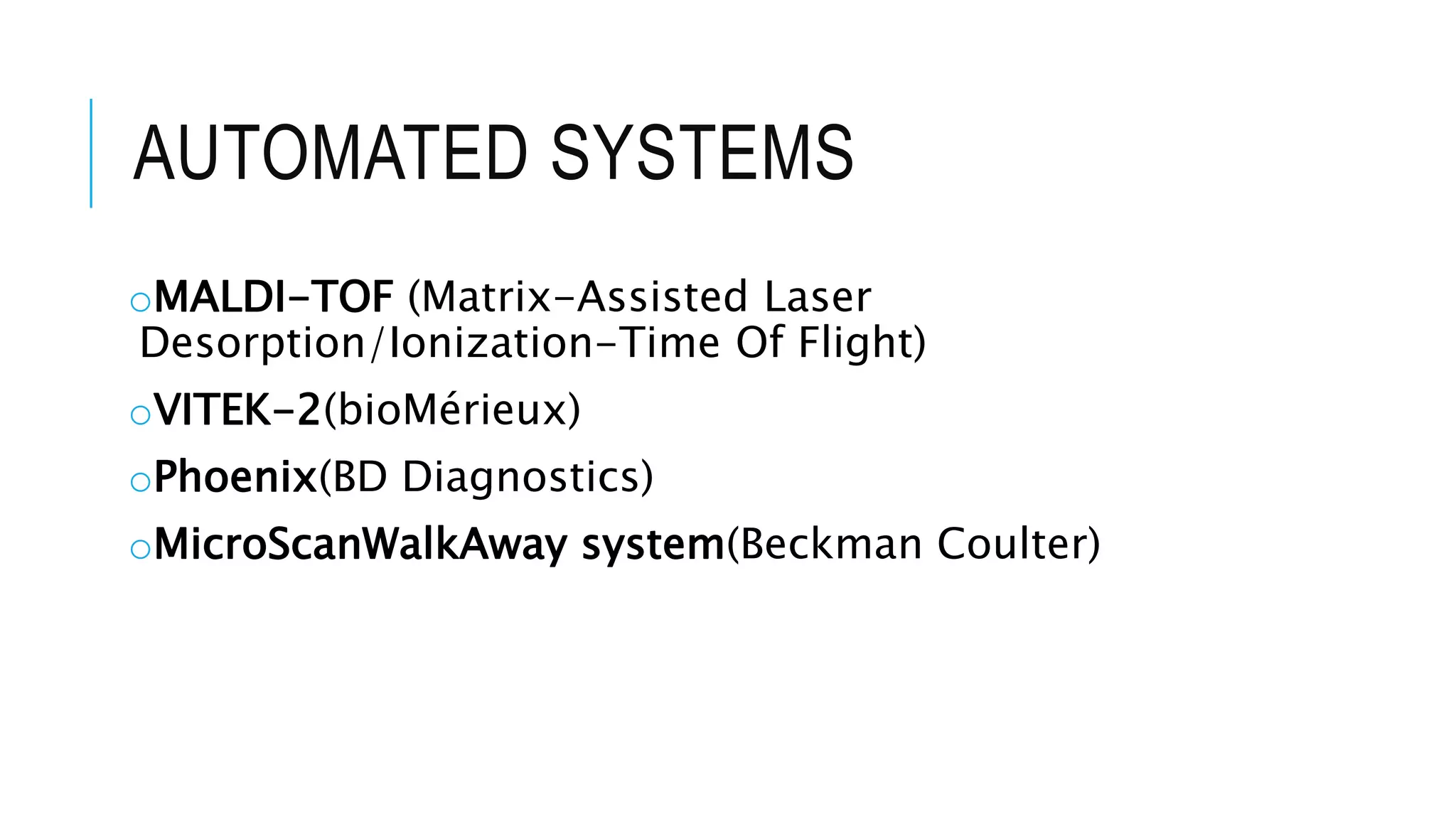
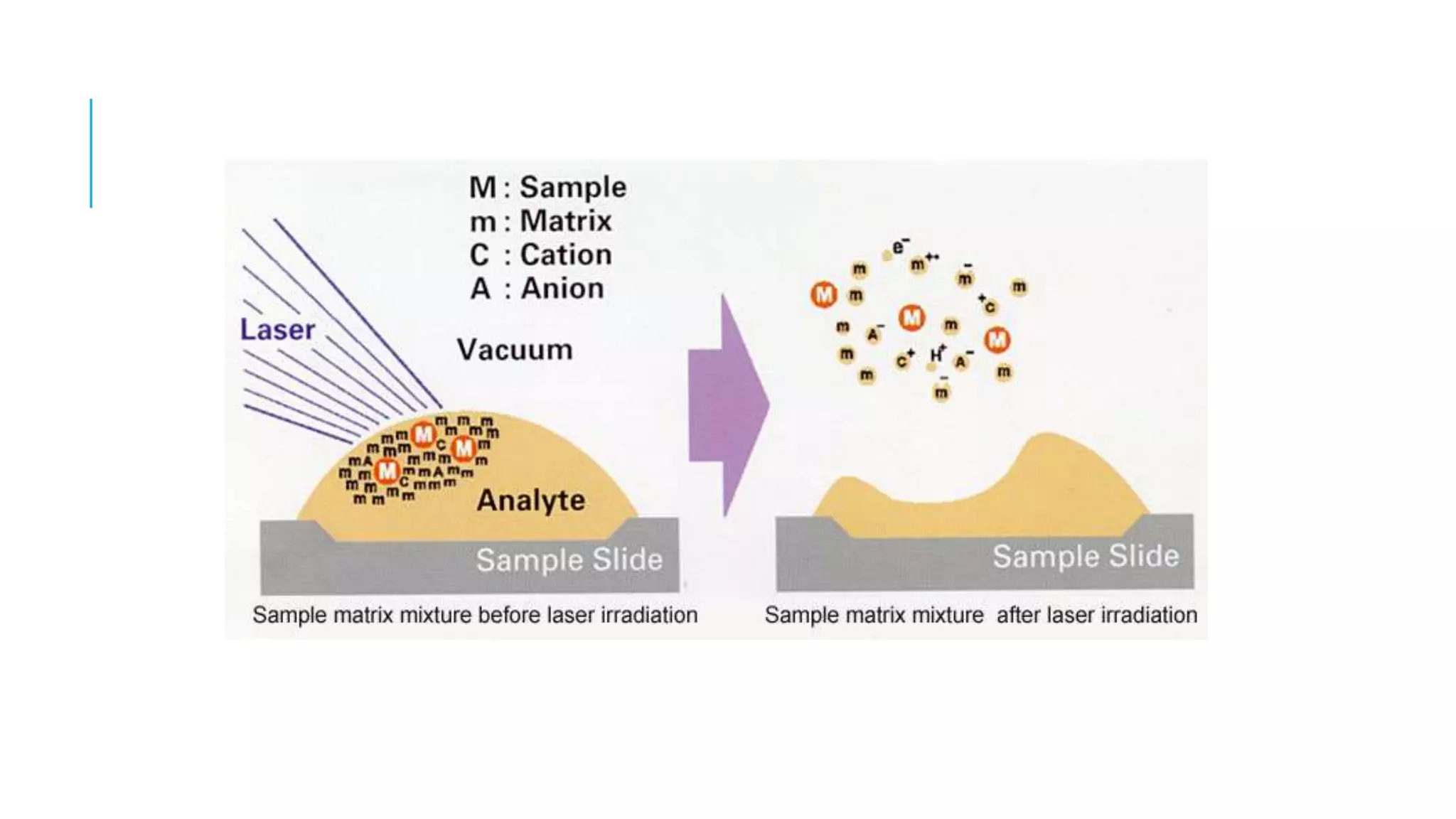
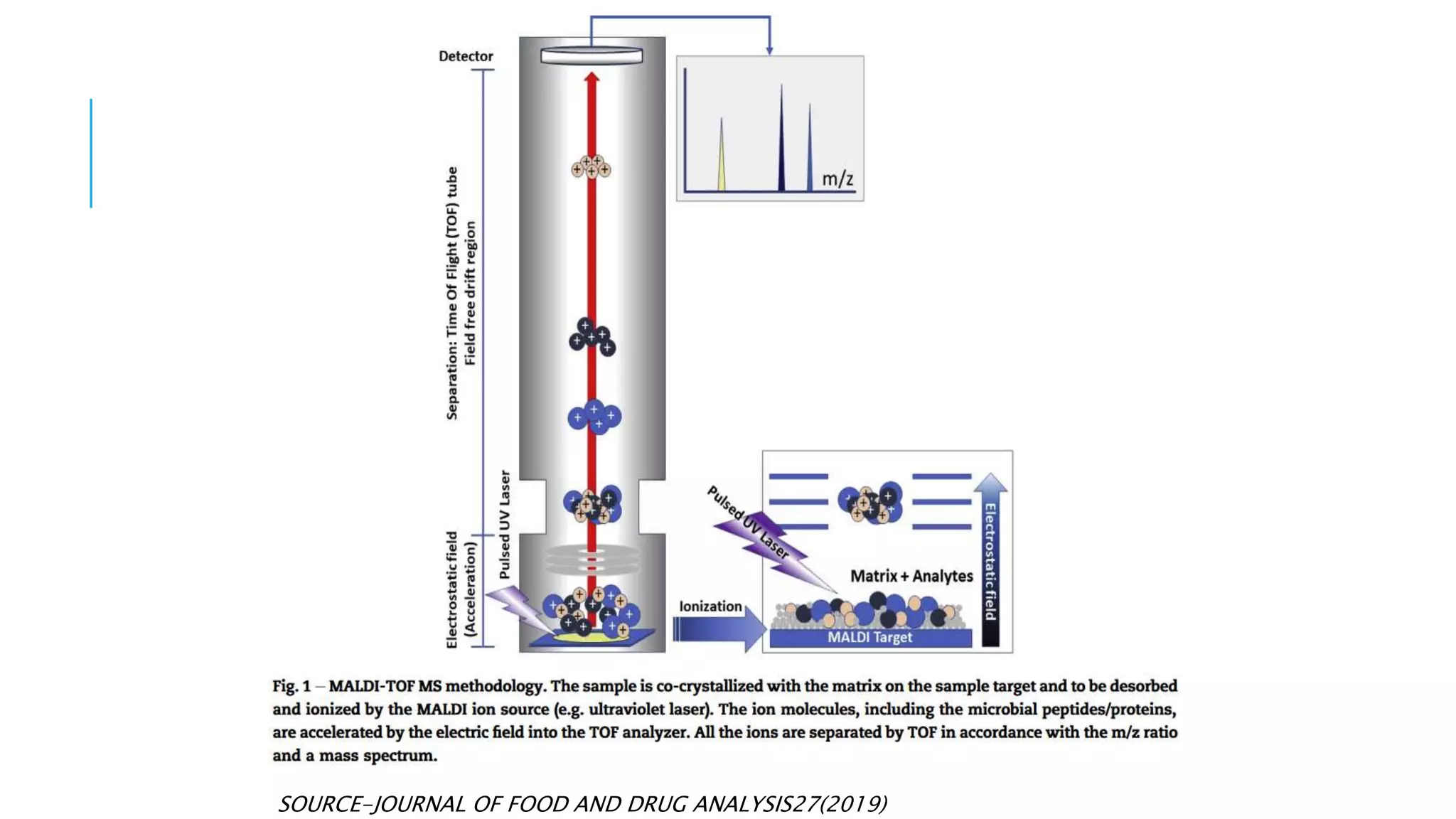
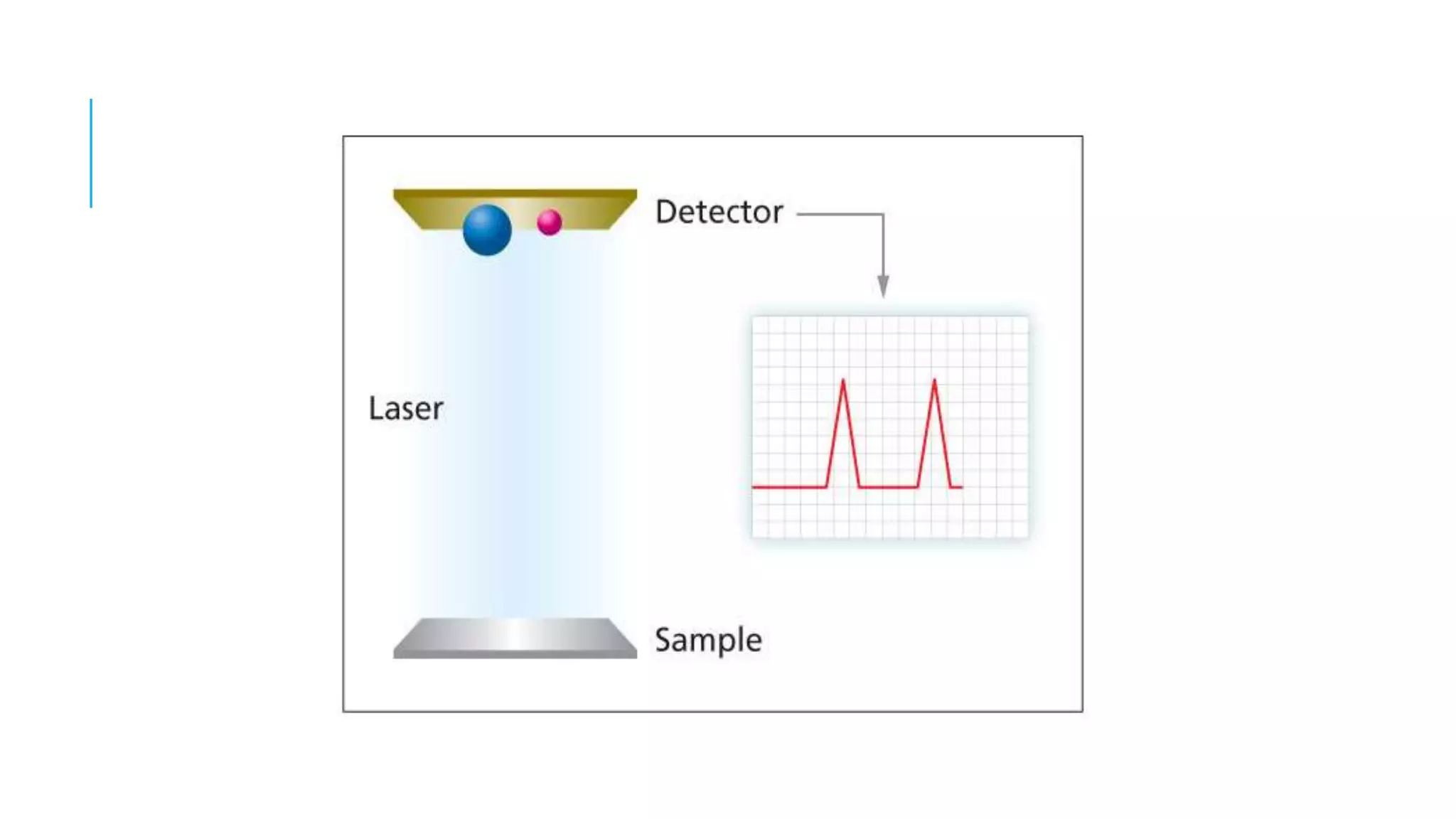
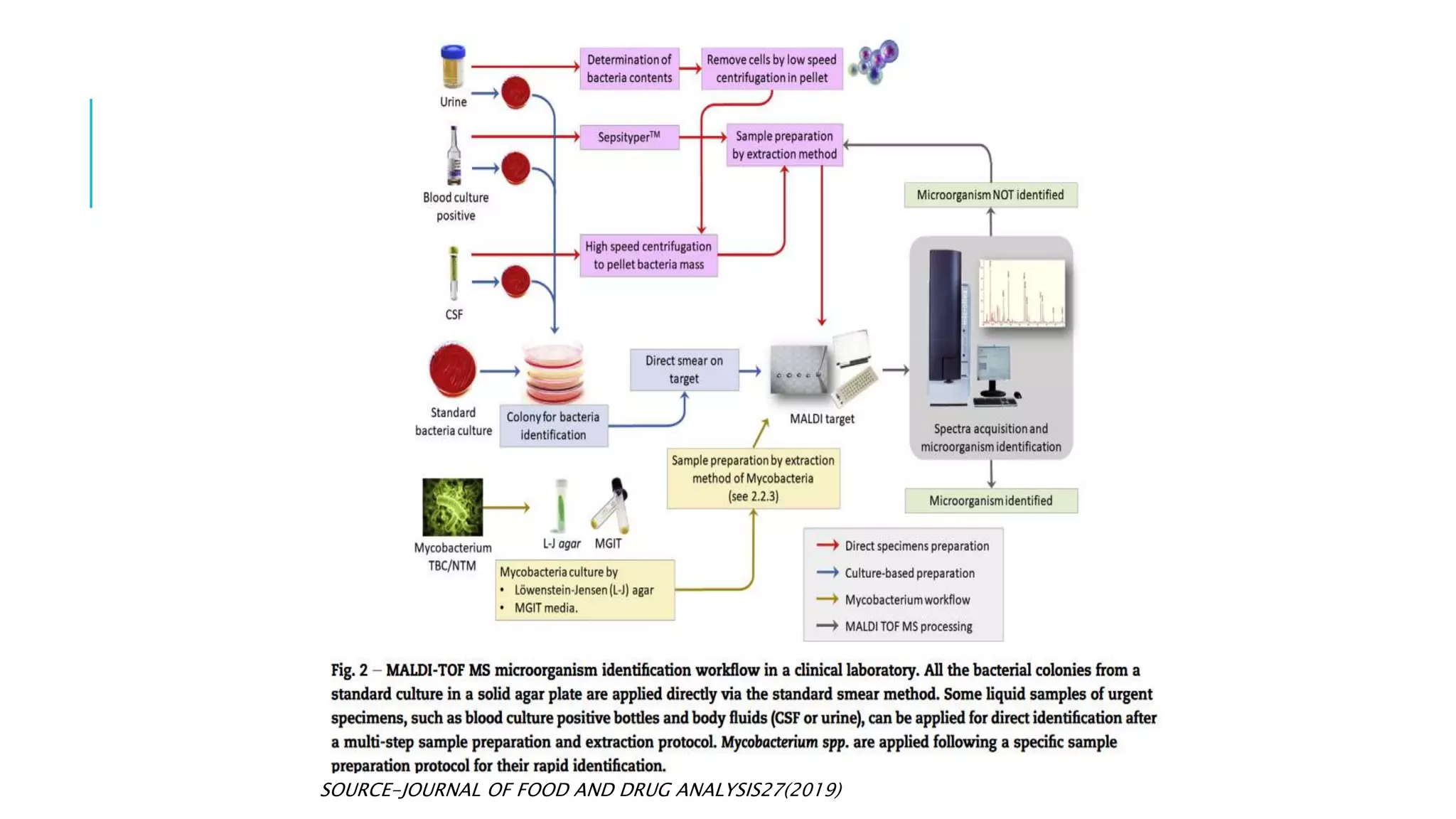
The document discusses various automated systems used for bacterial identification and antimicrobial susceptibility testing, which have evolved from traditional biochemical tests since their introduction in the 1970s. Key automated systems mentioned include MALDI-TOF, Vitek 2, Phoenix, and Microscan Walkaway, each offering rapid identification and testing with differing methodologies and turnaround times. These systems improve accuracy and efficiency in clinical microbiology laboratories by providing precise identification and susceptibility results within a few hours.