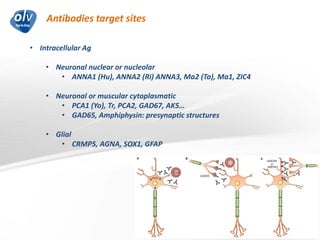
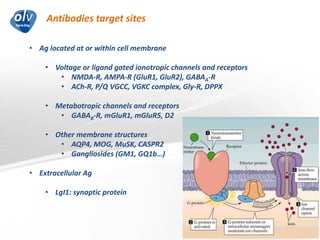
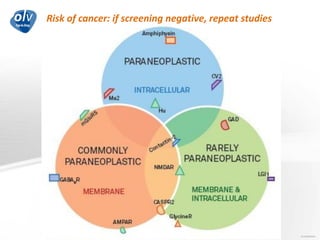
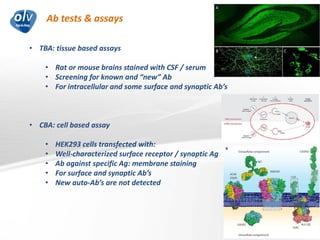
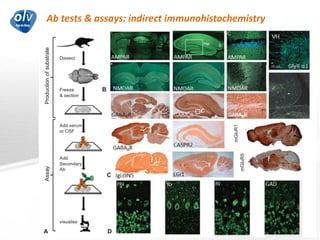
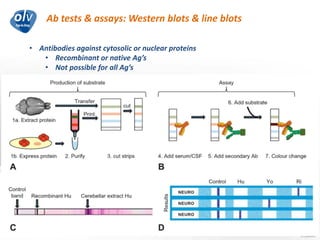
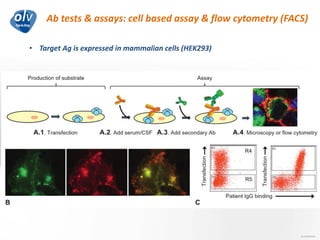
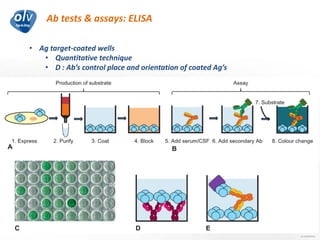
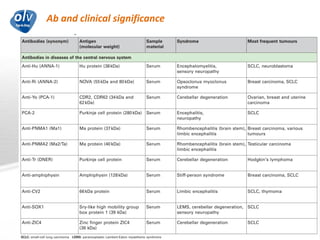
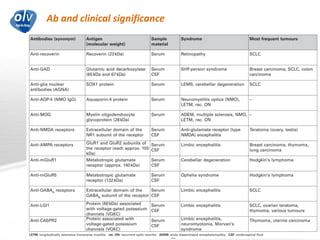
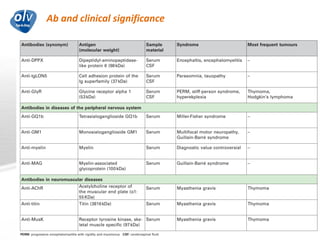
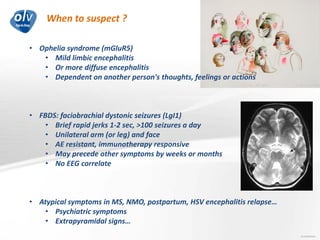
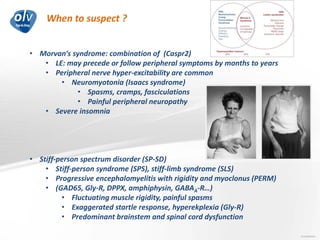
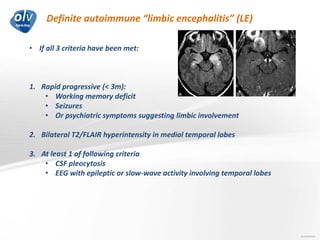
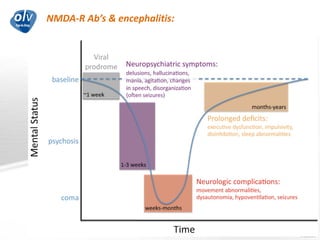
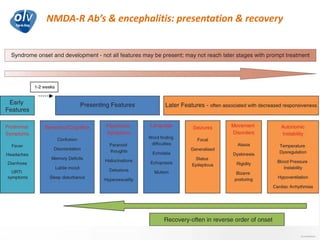
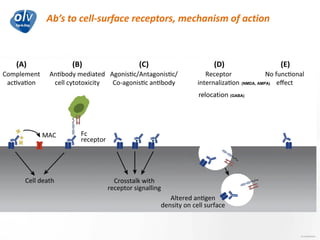
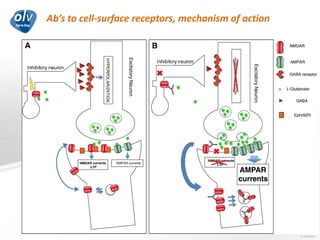
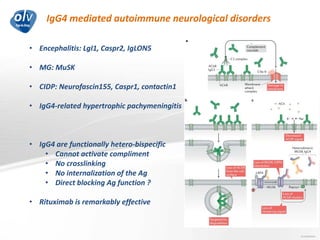
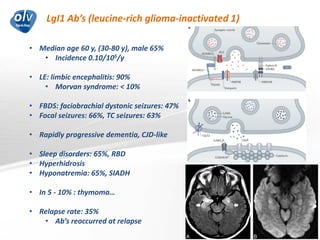
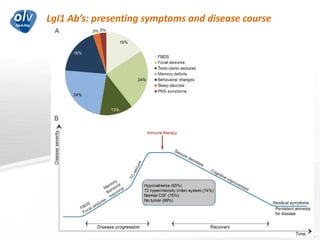
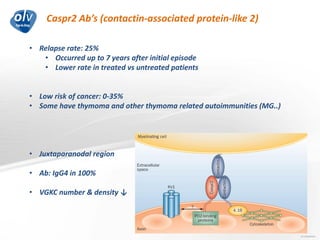
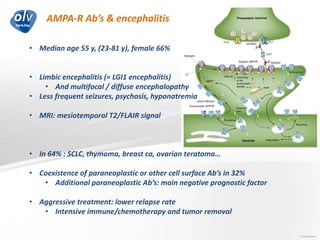
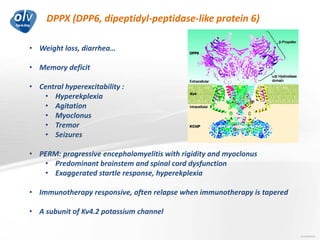
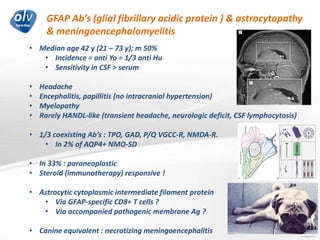
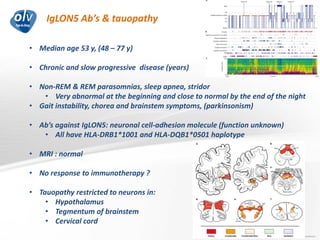
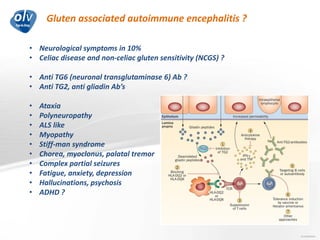
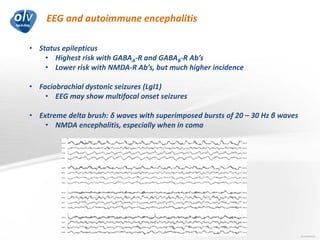
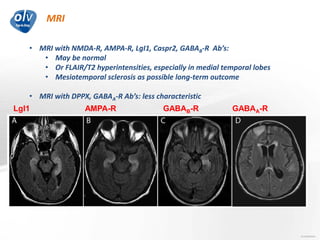
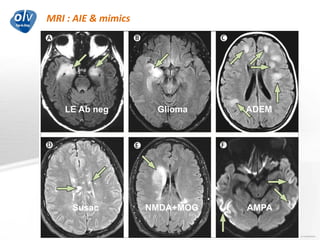
This document discusses various autoimmune encephalitis conditions, including those associated with antibodies against intracellular, synaptic, and cell surface antigens. Examples discussed include NMDA receptor antibody encephalitis, which commonly affects young women and is associated with ovarian teratomas. LGI1 and CASPR2 antibody encephalitis are described along with their clinical presentations and associations with thymomas. Stiff person spectrum disorders linked to glycine receptor and gephyrin antibodies are also summarized. Throughout, the document provides details on presentations, diagnostic criteria, treatment responses, and cancer associations for the different autoimmune encephalitis subtypes.