- ALCCaS is a multi-center randomized controlled trial comparing laparoscopic and open surgery for colon cancer in Australia and New Zealand that recruited 600 patients between 1999-2008.
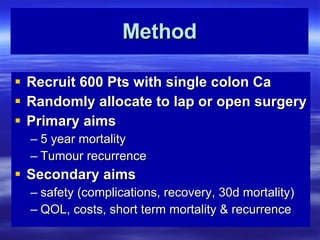
- The primary aims are to compare 5-year mortality and tumor recurrence rates between the two surgical methods. Secondary aims look at short-term outcomes like complications, recovery time, and costs.
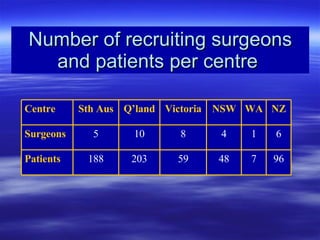
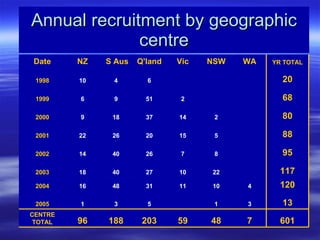
- Over the study period, 592 patients were recruited across 7 centers by 34 surgeons. Data collection is now complete and results will be analyzed to determine if laparoscopic surgery is as safe and effective as open surgery for colon cancer.