1) Early atomic models proposed atoms as billiard balls (Thomson) or plum puddings (Rutherford) until experiments showed atoms have a small, dense nucleus.
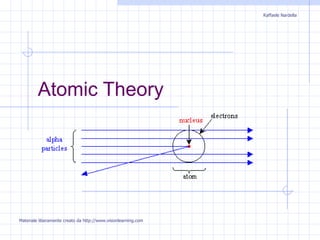
2) Rutherford discovered alpha particles bouncing off a gold foil, indicating a tiny nucleus.
3) Bohr incorporated Rutherford's nuclear model and proposed electrons orbit in distinct energy levels, explaining atomic emission spectra.