Atomic physics=Lectures from Dr. Parvez Ahmed Asstt. Prof., at INMAS, Rajshahi, Bangladesh.
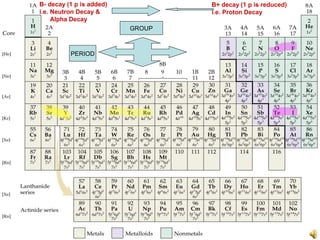
- 1. PERIOD GROUP B- decay (1 p is added) i.e. Neutron Decay & Alpha Decay B+ decay (1 p is reduced) i.e. Proton Decay
- 3. BASIC TERMS 1. smallest part of a chemical element that still retains the properties of the element=atom 2. substance that cannot be broken into subatomic fundamental particles by chemical processes= Element 3. That electrically neutral substance which is formed when atoms transfer or share their valence shell electrons with other atoms of same or different element (to complete their valence shells) = Molecule/Compound 3 KINDS OF MATTER=Elements, Compounds, Mixtures (A mixture is made from two or more elements, compounds or both - that are not chemically combined. Most common matter) Outermost shells are called Valence shells.
- 4. BASIC TERMS Electron Volt=used to express energy (Non-SI) of ion & subatomic particle-that have been accelerated in particle accelerator. One electron volt is equal to the amount of energy gained by an accelerating electron in vacuum using 1Volt electrical potential difference. One electron volt is equivalent to 1.60207 × 10-19 J. Outermost shells are called Valence shells.
- 5. BASIC TERMS When metal atoms combine with each other, the outermost/valence shell electrons lose contact with their parent atoms & move freely around the whole sample. These freely moving electrons, called conduction electrons, can carry heat energy and electric charge easily throughout the metal, making metals good conductors of heat & electricity. The remaining positively charged atomic centers form an ordered/crystalline structure. Outermost shells are called Valence shells.
- 6. BASIC TERMS ATOM NATURAL Hydrogen to Uranium (i.e. E 1-92) All matters in nature are formed by their combinations ARTIFICIAL Neptunium to (i.e. E 93-118)
- 7. BASIC TERMS ATOM May be stable, radioactive or Chemically reactive If chemically reactive Stable molecule CRB same atoms Stable compound CRB different atoms Free radical CRB different ions CRB=chemical reaction between
- 8. BASIC TERMS ATOM/ELEMENT LIGHT=3 lithium, beryllium, and boron (E 3-5) MEDIUM=4 carbon, nitrogen, oxygen, and fluorine (E 6-9) HEAVY=111 remainder of the Elements (Ne=10 & above) Light elements i.e. hydrogen, hydrogen’s isotope deuterium and helium are formed shortly after big bang. Elements heavier than helium and lighter than iron are formed in nuclear processes in stars, and Elements heavier than iron are formed in supernova explosions.
- 9. SPECTRUM OF EMR (after interacting with matter) (series of 6 colored component (i.e. visible light or simply, light)+ invisible radiations- arising from splitting of composite/white light when it is passed through a glass prism; In 1666, discovered by English Physicists Newton) Each component has separate range of wavelengths, thereby having separate range of refractions. E.g. violet light refracts more than red light, so when their mixture is passed through a glass prism-they show separate range of refractions. Rainbow is a similar, natural spectrum-produced by meteorological phenomena. Invisible radiations=during 19th century & later, radio/TV wave & microwave beyond infrared; X ray & gamma ray beyond ultraviolet was discovered inside spectrum. (Absorption & Emission)Spectroscopy=study of spectrum’s wavelengths absorbed or emitted by the sample being analyzed (originating after EMR-Matter interaction)-by using devices namely spectroscope (to produce & visually observe spectrum), spectrograph (to observe & photographically record spectrum) and spectrophotometer (to measure brightness of each 6 component colored light). In 1859, prism spectroscope was developed by German Physicists Kirchhoff & Bunsen for chemical analysis. Audio spectrum=noise analysis by splitting tones having different pitch. Mass spectrum=compound/isotope analysis by splitting their atoms having different mass number. Mass Spectrometer is used for such. EMR=energy bearing wave that always moves at the constant speed of about 300,000 km/sec (186,000 mi/sec) in vacuum & each wave inside spectrum of EMR arises from moving charged particle (i.e. electron, proton) and consists of changing electric & magnetic field.
- 10. SPECTRUM (series of 6 component colored/visible light+invisible radiations-arising from splitting of composite/white light when it is passed through a glass prism; In 1666, discovered by English Physicists Newton) After excitation, outer energy level electron emits visible, infrared, and ultraviolet; inner energy level electron emits x ray; nuclear energy level proton emits gamma ray. Quantum/Photon=particle-like, discrete packet of electromagnetic radiation. It is different from matter that it has no mass & always moves at the constant speed of about 300,000 km/sec(186,000 mi/sec) in vacuum and consists of changing electric & magnetic field.
- 11. SPECTROSCOPY (study of colors of light/spectrum;) Each element (having unique set of energy levels) absorbs/emits set of photons (having characteristic energies similar to an atom’s unique set of energy levels).
- 12. CHEMICAL ELEMENTS IN PERIODIC TABLE (developed independently by two chemists, in 1869 by the Russian Dmitry Mendeleyev and in 1870 by German Julius Lothar Meyer physical and chemical properties of the elements tend to recur at regular intervals of 2, 8, 18, and 32) Chemical element Metal (electropositive/reducing agent/electron donor) & Form +ion/Cation Non-metal (electronegative i.e. gains electron) & Form -ion/Anion
- 13. CHEMICAL ELEMENTS IN PERIODIC TABLE (developed independently by two chemists, in 1869 by the Russian Dmitry Mendeleyev and in 1870 by German Julius Lothar Meyer physical and chemical properties of the elements tend to recur at regular intervals of 2, 8, 18, and 32) Alkali metal=6 in number (in column 1, valence +1) & last one is radioactive. They are soft/malleable & highly reactive (i.e. remain in nature as compounds). Alkaline earth metal=6 in number (in column 2 , valence +2). They are semisoft/extrudable & reactive (mostly remain in nature as compounds). Transition metals/elements=40 in number (from column 3 to 12). They have incomplete penultimate electron shell, variable valences (+1 to +8), form bright colored compounds, highly conductive to heat and electricity but less reactive than previous 2 metal groups. They form both ionic and covalent bonds with anions They have high melting point & magnetic properties too. They act as catalyst in biochemical & industrial processes (e.g. plastic, petroleum.) Rare earth metals/Lanthanide series=14 elements incl. Holmium, Z 57 to 70. They are found in monazite mineral, separated from mineral by precipitation, separated from each other by ion-exchange method. They are paramagnetic (i.e. weakly magnetized), pyrophoric (i.e. ignitable in open air). They form alloy with other metals. They are trivalent except cerium (4+). Actinide series=First 4 natural radioactive (Z 89 to 92 incl. Uranium) + next 10 synthetic radioactive Transuranium (Z 89 to 102).
- 14. CHEMICAL ELEMENTS IN PERIODIC TABLE (developed independently by two chemists, in 1869 by the Russian Dmitry Mendeleyev and in 1870 by German Julius Lothar Meyer physical and chemical properties of the elements tend to recur at regular intervals of 2, 8, 18, and 32) Halogens=First 4 natural +synthetic & radioactive last 1 in number (in column 17, valence -1). They are highly reactive & form table salt like molecules with metal (e.g. sodium) and complex ions with both metal & nonmetals. Astatine behaves most like a metal & is highly carcinogenic. Noble gases=6 in number (in column 18) & last one radioactive.. They are commonly unreactive & all are gaseous at room temperature.
- 15. CHEMICAL ELEMENTS IN PERIODIC TABLE (developed independently by two chemists, in 1869 by the Russian Dmitry Mendeleyev and in 1870 by German Julius Lothar Meyer physical and chemical properties of the elements tend to recur at regular intervals of 2, 8, 18, and 32) 18 columns/groups/families 7 rows/periods (1 2,2 8,3 8,4 18,5 18,6 32,7 32+)
- 16. CHEMICAL ELEMENTS IN PERIODIC TABLE (developed independently by two chemists, in 1869 by the Russian Dmitry Mendeleyev and in 1870 by German Julius Lothar Meyer physical and chemical properties of the elements tend to recur at regular intervals of 2, 8, 18, and 32)
- 17. CHEMICAL ELEMENTS IN PERIODIC TABLE (stable nucleus must have a 'magic' number of neutrons or protons=82,114,126,184; Also 2,8,20,28,50) Heavy transuranium elements are produced in particle accelerators by bombarding atomic nuclei with charged atomic nuclei or nuclear particles (neutrons, electrons, protons, and alpha particles) to form a heavier element. These super heavy elements are radioactive and decay into more stable, lighter elements rapidly. It is extremely difficult to manufacture large quantities of the elements heavier than plutonium. This problem is being overcome by bombarding uranium and plutonium with very intense streams of neutrons in reactors Bismuth (Z 83) & onward elements decay radioactively. Hypothetical isotope of super heavy element 114 containing 184 neutrons may have a comparatively stable nuclear arrangement. Another island of stability has been theorized to exist around an isotope of element 126. Producing such heavy nuclei will require accelerating much heavier ions than have been accelerated to date.
- 18. UNSTABLE/RADIOACTIVE ELEMENTS (Excluding K-40, all isotopes of element 84 i.e. Polonium & onward are Radioisotopes) ISOTOPE/ NUCLIDE NATURAL ARTIFICIAL (Radioactive:>3000) Stable=280 i.e. it can exist by itself for a long period of time. Unstable /Radioactive=83
- 19. UNSTABLE/RADIOACTIVE ELEMENTS (Excluding K-40, all isotopes of element 84 i.e. Polonium & onward are Radioisotopes) Unstable nuclei created in the laboratory decay rapidly but natural ones decay more slowly. Natural ones were formed in the interiors of stars more than 5 billion years ago. They were part of the cloud of gas and dust & formed our solar system incl. our Earth & other planets. Decay of natural radioactive elements provides much of the energy that heats Earth’s core. Common (among 83) natural radioisotopes=potassium-40 (19 column 1), thorium-232 & uranium-238 (actinide series 90 & 92). To estimate amount of isotope presented when mineral was formed, then measure how much has decayed. Knowing the rate at which the isotope decays, they can determine how much time has passed. This process, known as radioactive dating. Only sodium, beryllium, aluminum, and phosphorus has no natural isotopes; There remains 280 natural stable isotopes on Earth.
- 20. History of Particle physics • 400 B.C.=Democritus gave idea about atom. • 1808=John Dalton gave idea about atom as indestructible. • 1896=H. Becquerel discovered radiation emitting nature (natural radioactivity) of Thorium & Uranium. • 1897=J.J. Thompson discovered electron. • 1900=Max Planck showed that energy radiates as discrete/particle form (origin of Quantum theory/mechanics). • 1911=Ernest Rutherford identified α,β & γ radiation and discovered nucleus. • 1913=Neils Bohr gave Bhor model of atom. • 1919=proton was discovered by Ernest Rutherford (with prior idea of Eugen Goldstein in 1885). • 1926=Wave model of atom was theorized by Louis Victor de Broglie, Schrödinger, Heisenberg etc. • 1927=Paul Dirac gave idea about anti-electron (positron) in atom. • 1932=First discovery of Atom probing accelerator (generator, cyclotron & after WW-ll=synchrotron) • 1932=positron (anti-particle of electron) & neutron were discovered by Carl D. Anderson (in 1936, he also discovered Meson, predicted by Hideki in 1935) & Chadwick (with prior idea of Ernest Rutherford about neutron in 1920) respectively. • 1942=Enrico Fermi led first controlled uranium fission reaction • 1945-Bombs dropped on Hiroshima and Nagasaki. • 1956= antineutrino & neutrino (lepton) (with prior idea of W. Pauli about neutrino in 1930) were discovered. • 1964-1995=Murray Gell-Mann & later Glashow lead to discovery of subsequent 6 quarks, other leptons & Force carrying particles (Bosons) in proton & neutron (Quark/Standard model of particle physics). • 1995-onward=research is going on to find graviton, mass yielding-Higgs boson, dark energy (so that theory of everything-TOE can be established) German physicist Arnold Sommerfeld’s pupils are Wolfgang Pauli, and Werner Heisenberg.
- 23. STANDARD MODEL OF PARTICLE PHYSICS The theory where elementary/fundamental particles and their interactions are described. Limitations- Does not explain why & how some particles get mass and other particles do not. Most important and most theoretically supported particle-Higgs boson’s existence would solve that problem Because, some physicists believe that Higgs boson carries mass between particles and its mass is expected to be relatively large compared to that of other elementary bosons. When gaining velocity near speed of light (e.g. 2.6x108 m/s), mass of a resting/stationary particle (i.e. resting mass) becomes double-According to theory of relativity. In nuclear reactions, such velocity of particle/energy is seen when mass of particle is converted into energy and vice versa.
- 24. STANDARD MODEL OF PARTICLE PHYSICS Sub Atomic Particles Excluding antiparticles 4 Stable i.e. e- ,electron neutrino, muon neutrino, & proton Unstable Remaining particles. In 1975, tau lepton by Physicists Martin Perl (USA) In 1983, W & Z bosons by Physicists Rubbia (Italy) & Meer (Holland) In 1995, top quark by Physicists in California, USA Among particles, heaviest particle is top quark=179 GeV/c2 In 1970, in California, USA- Discovery of 1st Gen. Quark, related antiquark & gluon-using high-powered e- beam to proton
- 25. MASS & WEIGHT;VELOCITY & ACCELERATION (System=MKS/SI, CGS,FPS) Mass=total amount of particle in a system/body. It imparts resistance to motion (of that mass bearing particle). Weight=total amount of gravitational force on a given mass. It depends on distance/range of that given mass from gravitational force carrying object. E.g. in interstellar space, weight is nearly zero. Velocity=rate of change of distance/position per unit of time (i.e. magnitude/speed) in particular direction. E.g. meter per second in particular direction. Acceleration=rate of change of velocity per unit of time. E.g. meter per second per second. When gaining velocity near speed of light (e.g. 2.6x108 m/s), mass of a resting/stationary particle (i.e. resting mass) becomes double-According to theory of relativity. In nuclear reactions, such velocity of particle/energy is seen when mass of particle is converted into energy and vice versa.
- 26. SPECIAL RELATIVITY (In special theory (E=mc2 )-when & how matter behaves as its velocity approaches the speed of light and vice versa) There is no ultimate reference space/point/place in the micro- or macro-world, where one can stand and give the final word on how fast everything is moving in relation to everything else i.e. all frames of reference are equivalent, and calculations of velocities made from any frame must also be equivalent. Equivalence of mass and energy: Assuming that the speed of light in a vacuum is constant, and that physical laws have the same mathematical form throughout the universe, when gaining velocity near speed of light (e.g. 2.6x108 m/s), mass of a resting/stationary particle (i.e. resting mass) becomes double. When gaining velocity near speed of light (e.g. 2.6x108 m/s), mass of a resting/stationary particle (i.e. resting mass) becomes double-According to theory of relativity. In nuclear reactions, such velocity of particle/energy is seen when mass of particle is converted into energy and vice versa.
- 27. RELATIVITY (In special theory (E=mc2 )-when & how matter behaves as its velocity approaches the speed of light and vice versa) There is no ultimate reference space/point/place in the micro- or macro-world, where one can stand and give the final word on how fast everything is moving in relation to everything else i.e. all frames of reference are equivalent, and calculations of velocities made from any frame must also be equivalent. Einstein’s Photoelectric effect Photoelectric effect=low-energy photon strikes an atom & ejects (If the photon is sufficiently energetic) an inner energy level electron from the atom (proving in 1905 that electromagnetic radiation has particle properties, thus contributed to development of quantum theory). Energy of the ejected electron depends on the frequency of striking/incident photon. Use-in photoelectric cell, where e- ejected from photocathode & moves to anode, under the influence of an electric field. When gaining velocity near speed of light (e.g. 2.6x108 m/s), mass of a resting/stationary particle (i.e. resting mass) becomes double-According to theory of relativity. In nuclear reactions, such velocity of particle/energy is seen when mass of particle is converted into energy and vice versa.
- 28. QED (define interaction between charged particle & EM energy) QED (quantum electro dynamics) superseded the 1860’s classical wave theory of electromagnetic radiation-which was proposed by British physicist James Clerk Maxwell in 1860. In 1860, Maxwell successfully predicted that light is a EM wave. In 1900, Planck predicted that light radiates as ‘Quantum’ or discrete unit. In 1905, Einstein proved Planck’s theory by his work on ‘photoelectricity’ and gave E=mc2 (special theory of Relativity) assuming particle-wave dual nature in both matter & energy. 3 Interactions of EM energy with matter: Photoelectric effect=low-energy photon strikes an atom & ejects (If the photon is sufficiently energetic) an inner energy level electron from the atom (proving in 1905 that electromagnetic radiation has particle properties, thus contributed to development of quantum theory). Compton effect/scattering=medium-energy photon strikes an atom’s electron & then both particles (i.e. e- & incident/striking photon) scatter/deflect at an angle to the direction of the path of striking photon. Incident photon, giving some of its energy to the electron, emits with a longer wavelength and lower energy (proving in 1922 that electromagnetic radiation has both wave and particle properties, a central principle of quantum theory). Pair production=very high-energy (at least 1.2 Mev) photon strikes element of high atomic weight & a pair of inner energy level electrons (e- & e+ ) are produced and is ejected (If the photon is sufficiently energetic) from the atom in opposite/divergent direction (thus providing an example of conversion of energy into mass).
- 29. QED (define interaction between charged particle & EM energy) Phases of QED/ Quantum mechanics/ Quantum theory/Wave-particle duality First/Old phase/element=1900-1922 By Planck, Einstein, Neils Bohr & Arnold Sommerfeld. New/Joined QED-Special relativity= 1926-1950, by Dirac, Heisenberg, Pauli etc etc. According to wave-particle duality, both particle & forces’ wave must have momentum, discrete/fixed wavelength/frequency and energy. Wave-particle duality confirms that wave of a given wavelength correspond to particle of a given momentum. Planck’s constant is product of wavelength & momentum.
- 30. A universal constant of nature/Planck’s constant (h) [energy of emitted photon E equals frequency of emitted photon f multiplied by h] In 1916-Robert Millikan (USA) successfully measured its value (6.626 × 10-34 joule-second in MKS system). Wave-particle duality confirms that wave of a given wavelength correspond to particle of a given momentum. Planck’s constant is product of wavelength & momentum. Energy of Photon is equal to the frequency of Photon multiplied by Planck’s constant, i.e. E=f x h.
- 31. Electromagnetic spectrum (made by many waves having)
- 32. Intrinsic AM/Spin (Unit is h/2π, where h is Planck’s constant, Leptons have spins of +1/2) Spin is the measurement of rotation of a particle. Rotation is measured in multiples of the constant number h/2p, where h is a number called Planck’s constant, equal to 6.626 × 10-34 joules-sec. In 1925, Dutch-born American physicists Goudsmit and Uhlenbeck added 4th quantum number (spin of electron) to provide complete explanation of atomic spectra. Later, this number was extended to all particles, namely proton, neutron, nucleus, anti- particles. It is measured as integer or half-integer multiples of unit h/2π, Fermions (i.e. proton, neutron, electron) have odd half-integer spin (1/2, 3/2,...); Bosons (i.e. photon, alpha particle, meson) have whole integer spin (0,1,...). Angular/Rotational Momentum Product of mass & rotation velocity; In Atomic/Particle Physics, it is combined by : Intrinsic/Spin i.e. rotation around its axis; Unit is h/2π Orbital results from motion of spinning particle. or=rotating section of a chine. pes of Rotation/Circular vement/Gyration- nning & Orbiting.
- 33. Atoms are composed of 2 regions: Nucleus: center of the atom that contains the mass of the atom Electron: electronic waves carrying definite energy create regions of negative electric charge (called orbital) around positively charged nucleus. Electrons have no real size & have zero radius. 24 Fermions (Fundamental):6 quarks+ 6 leptons, with their anti-particles (i.e. electron, proton, neutron etc.) 6 Bosons (Fundamental): force carrying particles (photon, gluon, W &Z weak force particles + Higgs boson, graviton) 6 LEPTONS electronic muonic tauonic charged electron muon tau neutral neutrino muon neutrino Tau neutrino ATOMIC STRUCTURE (Subatomic particles are classified on mass, spin & electric charge; Fundamental particles form both matter & energy in Universe) In1936,Physicistsfinalized these2types:
- 34. 6 LEPTONS electronic muonic tauonic charged electron muon tau neutral electron neutrino muon neutrino Tau neutrino FUNDAMRNTAL LEPTONS (History Range=In 1897, e- by J.J. Thompson & In 1995, top quark by Physicists in California, USA) 1st Generation/Group Leptons (Found in everyday matter): e- =stable fundamental particle having electric charge -1(i.e. 1.602 × 10-19 coulomb ) & mass 511 keV/speed of light squared (Physicists use this unit because mass of particles is small). They have no detectable shape or structure. They form layer(s) surrounding positively charged nucleus to make the whole atom electrically neutral. It can spin clockwise or counterclockwise making it acting as a tiny magnet. It is affected by electromagnetic force & gravitation, as it has electric charge & mass respectively. Number of electrons in an uncharged atom must be equal to the number of protons, and the arrangement of these electrons determines the chemical properties of the atom. Uses of e- : 1.Used for electrical devices, 2.(By using e- beam) For producing TV picture & X ray, 3.(By using e- beam) For illuminating study object (e.g. virus) in electron microscope. 4.(By using high-energy e- beam) For revealing elementary particles such as protons, neutrons, and even quarks. Fundamental particles, meaning they cannot be broken down further= provide basic units of matter and energy in the universe.
- 35. 6 LEPTONS electronic muonic tauonic charged electron muon tau neutral electron neutrino muon neutrino Tau neutrino 1ST GEN. NEUTRINOS 1st Generation/Group Leptons (Found in everyday matter): electron neutrino= stable neutral fundamental particle having little mass not yet detected. It is so small that it passes right through most material including earth. It interacts rarely with other particles because of their tiny size, neutral electric charge, and very high speed. It travels in all medium equally at light’s speed in vacuum. It comes mainly from Sun (@ 70 billion/cm2 /second on Earth’s surface), from collisions in the atmosphere, and from beta decay of radioactive elements on Earth. All neutrinos have a spin of +1/2. Second and Third generation Leptons are unstable- i.e. they exist for only a fraction of a second before decaying into stable First generation Leptons. Fundamental particles, meaning they cannot be broken down further= provide basic units of matter and energy in the universe. Leptons have spin of +1/2.
- 36. 6 LEPTONS electronic muonic tauonic charged electron muon tau neutral electron neutrino muon neutrino Tau neutrino 2ND & 3RD GEN. NEUTRINOS 2nd Generation/Group Leptons (Found during high-energy collisions between particles seen in Earth’s atmosphere by cosmic particles, in Particle Accelerator, in stellar explosion- i.e. supernova etc): Muon= has electric charge –1 and mass 106 MeV/c2 , i.e. much heavier than electron. Muon neutrino=has no electric charge and little mass not yet detected (Like other neutrinos). 3rd Generation/Group Leptons (Found during high-energy collisions between particles seen in Earth’s atmosphere by cosmic particles, in Particle Accelerator, in stellar explosion- i.e. supernova etc): tau= has electric charge–1 and mass 1.77 GeV/c2 , i.e. twice that of neutron-which is the heaviest particle in an atom tau neutrino=has no electric charge and little mass not yet detected (Like other neutrinos). Second and Third generation Leptons are unstable- i.e. they exist for only a fraction of a second before decaying into stable First generation Leptons. Leptons have spin of +1/2.
- 37. 6 LEPTONS electronic muonic tauonic charged electron muon tau neutral electron neutrino muon neutrino Tau neutrino HISTORY OF NEUTRINOS History of Neutrino: In 1920=while studying beta decay, neutrino is theorized. In 1930=W.Pauli suggested existence of neutrino. In 1940=muon was discovered. In 1956=American physicists Reines and Cowan discovered electron neutrino with its antiparticle. In 1962=American physicists Lederman, Schwartz & Steinberger discovered muon neutrino during pion decay. In 1970=First detection of solar neutrino by first using solar neutrino detector in south Dakota,USA. In 1975=American physicist Martin Perl discovered tau. In 1990=At its end, tau neutrino’s existence was indicated with great precision-it is not directly detected. In 1998=Japanese physicists neutrinos oscillate/convert into other neutrinos (proving existence of their mass). Fundamental particles, meaning they cannot be broken down further= provide basic units of matter and energy in the universe. Leptons have spin of +1/2.
- 38. NEUTRINO DETECTOR Neutrino Detector-filled by Cl/Ga; Based on its rare interaction with particle Underground e.g. on ocean floor, under polar ice to detect SOLAR electron & muon neutrino. Within reactor or PA To detect muon & tau neutrino By getting their interaction by-product= Muon & Tau. PA=Particle Accelerator RARE INTERACTIONS OF NEUTRINOS: 1. When electron neutrino combines with nucleus’ neutron to produce a proton, electron, and energy (reverse beta- decay), that atom’s atomic number goes up, changing it into a different element. 2. When muon neutrino interacts with nuclei, the collision produces charged muon. 3. When tau neutrino interacts with nuclei, it produces charged tau.
- 39. 6 LEPTONS electronic muonic tauonic charged electron muon tau neutral electron neutrino muon neutrino Tau neutrino CHERENKOV EFFECT/RADIATION (In 1934, Russian Physicist Pavel Cherenkov discovered this photoluminescence during his study on γ ray, i.e. Photon-transparent nonconducting liquid interaction) Fundamental particles, meaning they cannot be broken down further= provide basic units of matter and energy in the universe. Leptons have spin of +1/2. Neutrino Detector embedded/surrounded by water/ice depends on Cherenkov radiation which is flash of bluish light created from high-energy charged particles like electron, muon, tau (which are produced during neutrino-matter interaction) moving through water/ice (transparent nonconducting liquid ) at a speed greater than speed of light in water/ice. i.e. emission of bluish light created from high-energy charged particles moving through transparent nonconducting liquid (water, ice) or solid (e.g. plastic) material at a speed greater than speed of light in the same medium. Actually If a high-energy charged particle enters transparent nonconducting/dielectric/ insulating medium at a speed nearly that of light (c), its (charged particle) speed can then exceed c in that medium. Because speed of light (c) in that type of medium is reduced to speed= c/n, where n is the refractive index of the medium.
- 40. USES OF ELECTRON-1ST GEN. CHARGED LEPTON 1st Generation/Group Leptons (Found in everyday matter): e- : 1. Used for electrical devices, 2. (By using e- beam) For producing TV picture & X ray, 3. (By using e- beam) For illuminating study object (e.g. virus) in electron microscope. 4. (By using high-energy e- beam) For revealing elementary particles such as protons, neutrons, and even quarks. Ordinary, visible light can only resolute/create image of those objects which are larger than the wavelength of the light waves illuminating them. Wavelength of the visible light is millionth of a meter. So for smaller objects, visible light scatters/throws randomly off the object and do not reveal its shape. But Electrons have smaller wavelengths than visible light and thereby reveal smaller objects which are larger than the wavelength of e- waves illuminating them. For producing TV picture= e- beam is produced in vacuum CRT/Picture tube where negatively charged metal (i.e. cathode) is heated from which e- emits & accelerates toward positively charged metal (i.e. anode)
- 41. COSMIC RAY (i.e. Extraterrestrial Particles) (this name is given by American Physicist Robert A. Millikan, who also determined value of Planck’s constant ‘h’) Cosmic Particles 87% Proton 12% Alpha particle Others- heavy elements etc. Cosmic ray= Fast-moving subatomic particles that enter earth's atmosphere from outer space. High-energy (as a result of their high speed) cosmic particles produce secondary particles (e.g. electron, muon & gamma ray) by interacting with Earth’s atmospheric atoms. Source-exact source in outer space is still unknown, but interstellar (e.g. X ray binary star system) or intergalactic space is believed to be source of high-energy cosmic particles. In 1912- Austrian-American physicist Victor Franz Hess showed that Earth’s atmospheric ionization occurs due to cosmic particles. And the intensity of this ionization by electrically charged cosmic particles depends on latitude implying that those particles are deflected by Earth’s magnetic field. Detectors are tagged on high-altitude balloon or spacecraft (to get outside the atmosphere) to detect electric charge, mass, and energy of cosmic particles.
- 42. COMIC RAY INTERACTION Cosmic ray-Earth atm.’s Atom interaction (30 pions/60 neutrinos) 25% Neutral Pion 75% Charged Pion Decays to form- + Pion=antimuon (positron & electron neutrino) & muon neutrino - Pion=muon (electron & electron antineutrino) & muon antineutrino Cosmic particles produce secondary particles (e.g. electron, muon & gamma ray) by interacting with Earth’s atmospheric atoms Pion is made up of Strange quark, Hence pion is unstable, i.e. decays.
- 43. STELLAR (i.e. involving Star) EVOLUTION
- 44. STELLAR (i.e. involving Star) EVOLUTION Thermonuclear fusion occurs when intense heat and gravitational force cause hydrogen atoms together Nebula=cloud of interstellar gas and dust which, by gravitational force, coalesce to form protostar. Protostar=A compact/dense form with rising temperature & pressure at its core. Star=when rising temperature reaches 10 million degrees C (18 million degrees F), nuclear fusion (i.e. core’s hydrogen turns into helium) in the core starts, releasing tremendous energy as light (i.e. EMR) & heat. Red giant=After exhaustion of core’s hydrogen fuel, hydrogen from pericentric layer takes part in nuclear fusion-resulting expansion of outer layer (i.e. gas & dust). Nebula=After exhaustion of pericentric layer’s hydrogen fuel, inward collapse of red giant by gravitation occurs & ejection of its outer layer (i.e. gas & dust) in space is seen. White dwarf=After exhaustion of pericentric layer’s hydrogen fuel, inward collapse of red giant by gravitation occurs but may glow if remarkable amount of energy is trapped in its core. It may be of Earth’s size but remains extremely compact. Black dwarf=After release of all trapped energy, it becomes cold & glowless (i.e. black). Supergiant=After exhaustion of pericentric layer’s hydrogen fuel, core’s helium takes part in nuclear fusion to form carbon-resulting massive expansion of outer layer (i.e. gas & dust). Gravitational collapse & intense heat causes carbon to convert finally into iron.
- 45. Supernova=After exhaustion of all fuel, inward collapse of supergiant by gravitation occurs & subsequent explosion in outer layer is seen destroying this star totally or keeping its core intact only. Neutron star/Pulsar (as it emits pulsating radio waves)=After supernova phenomena, inside iron core’s atomic electrons & protons merge to form neutron-making the core extremely dense. (this star is first theorized by American astrophysicist Chandrasekhar in 1934) Black hole= After supernova phenomena, spared intact iron core is collapsed by gravitational pull-resulting a central super dense black object which attracts everything around it. STELLAR (i.e. involving Star) EVOLUTION Thermonuclear fusion occurs when intense heat and gravitational force cause hydrogen atoms together Quasar (quasi-stellar)=extremely remote astronomical compact object with very high energy output. Galaxy=group/assembly of stars, their planets & nebula-held together by gravitation. They may be spiral, elliptical or asymmetric. Strength of gravitational pull depends on mass of atom; Mass of atom is raised by nuclear fusion, induced by intense heat & pressure.
- 46. QUARKS-OTHER FUNDAMENTAL FERMIONS (Any quark or antiquark can have any color charge i.e. Color charges (having 3 color values & 3 anticolor values), via GLUON- among quarks or antiquarks are constantly changing, thereby holding the identical quarks or antiquarks together) 6 QUARKS 1st Gen. 2nd Gen. 3rd Gen. + ⅔ Up Charm Top - ⅓ Down Strange Bottom In 1964, American physicists Murray Gell-Mann and George Zweig classified proposed quarks as fermions Antiquarks are fermions whose electric charge and color charge are opposite from those of quarks. Color charge (both color & anticolor values) results Strong force. HISTORY OF QUARK DISCOVERY In 1950, Proton has cloudlike shell surrounding dense core- discovered by using high-powered e- beam to proton. In 1964, Gell-Mann & Zweig theorized for up & down quark; Strange quark was discovered. In 1970, in California, USA-First discovery of 1st Gen. Quark, related antiquark & gluon-using high-powered e- beam to proton. In 1974, Charm quark was discovered in California, USA. In 1977, Bottom quark was discovered. In 1995, Top quark was discovered in California, USA.
- 47. QUARKS-OTHER FUNDAMENTAL FERMIONS (Any quark or antiquark can have any color charge i.e. Color charges (having 3 color values & 3 anticolor values), via GLUON- among quarks or antiquarks are constantly changing, thereby holding the identical quarks or antiquarks together) Quarks=unlike Leptons, they have fractions of fundamental electric charge. They are always found in pairs (i.e. in mesons) or triplets (i.e. in baryons). Quarks have ½ spin. 1st Gen. Quarks (found in ordinary/every day matter; They form proton & neutron-which make up atoms’ nuclei)= Up type: has electric charge + ⅔ and mass between 1.5 and 5 MeV/c2 . Down type: has electric charge - ⅓ and mass between 3 and 9 MeV/c2 . 2nd & 3rd Gen. Quarks (found in cosmic particles & produced in laboratory resp.)= Charm type: has electric charge + ⅔ and mass between 1.1 and 1.4 MeV/c2 . Strange type: has electric charge - ⅓ and mass between 60 and 70 MeV/c2 . Top type: has electric charge + ⅔ and mass between 170 GeV/c2 . Bottom type: has electric charge - ⅓ and mass between 4.1 and 4.4 GeV/c2 . 3rd Gen. quarks are heaviest quarks. 6 QUARKS 1st Gen. 2nd Gen. 3rd Gen. + ⅔ Up Charm Top - ⅓ Down Strange Bottom In 1964, American physicists Murray Gell-Mann and George Zweig classified proposed quarks as fermions Antiquarks are fermions whose electric charge and color charge are opposite from those of quarks. Color charge (both color & anticolor values) results Strong force.
- 48. COMPOSITE FERMION (i.e. BARYON) (It contains odd number of elementary/fundamental fermions, e.g. triplet of quarks form proton, neutron) Proton= Stable & positively charged (+1) subatomic particle. It, with neutron form nucleus of atom. Mass of the proton is 938 MeV/c2 to 1 GeV/c2 . Its number in the nucleus determines type of chemical element. It is made of three quarks (2 up quarks & 1 down quark). It is affected by all four fundamental forces. Neutron= Electrically neutral, subatomic particle that forms, other than proton, part of atomic nucleus. Mass of the neutron is 940 MeV/c2 . It is slightly magnetic and when isolated from nuclear matter, it decays into proton, electron along with releasing energy. It is made of three quarks (2 down quarks & 1 up quark). Certain combinations of neutrons with protons in atomic nucleus make nucleus stable. E.g. if the nucleus has too many neutrons—one or more neutrons tend to decay. It is affected by all four fundamental forces. Other baryons are lambda & sigma particle which are formed by 2nd & 3rd Gen. quarks; These baryons are not found in atomic nucleus & are Unstable.
- 49. HISTORY OF BOSONS (History Range=In 1920, photon by J.J. Thompson & In 1983, W & Z bosons by Physicists Rubbia & Meer) History of Boson: In 1905=Planck & Einstein suggested existence of photon (confirmed In early 1920-by experiments) In 1924=Bose & Einstein set rules that define behavior of bosons In 1925=Pauli developed exclusion principle, In 1926=Enrico Fermi set rules that define behavior of fermions In 1934=Japanese physicist Yukawa Hideki suggested existence of pion/pi-meson (confirmed In 1947-by British physicist Cecil Powell). In 1964=British physicist Peter Higgs suggested existence of the Higgs boson. In 1970=Gluon (& 1st Gen. quarks) was discovered in California, USA. In 1983=Italian physicist Carlo Rubbia and Dutch physicist Simon Van der Meer discovered W and Z bosons. Searches continue for graviton, Higgs boson etc. laser and superfluidity of helium at low temperatures obey Bose-Einstein statistics
- 50. 4 FUNDAMENTAL FORCES (Forces that govern all interactions between particles and energy in the universe) Electromagnetic force= It (i.e. photon) arises from electrically charged or magnetic particle. It results repulsion between same charged particles & attraction between opposite charged particles. Repulsive force is 100 million times stronger than attractive force in atomic nucleus. Strong nuclear force= It (i.e. gluon) binds quarks together in hadrons and binds hadrons to one another by constantly changing quarks’ color charge, overcoming the electromagnetic repulsive force. Gravitational force= It causes attraction between any mass bearing objects. It is weak when the masses are small, but can become very strong when the masses are great. Weak nuclear force= It (i.e. W & Z bosons) governs how heavier fundamental or composite fermions decay/break up into other particles. Fundamental force= that govern the interaction between particles and energy in the universe Gravitational force is the weakest-on the scale of elementary particles and atoms.
- 51. FUNDAMENTAL BOSONS (In 1924, Indian physicist Satyendra Nath Bose, with Einstein developed Bose-Einstein statistics that defined properties of Boson particles) Unlike fermions, two identical bosons can occupy the same space. Fermions have odd half-integer spin (1/2, 3/2,...);but bosons have whole integer spin (0,1,...). 4 Elementary/Fundamental bosons (called mediators): Photon=carry EM force/energy which control interactions between charged particles. Gluon=carry Strong force which (by creating color charge) holds quarks together (which form hadrons-i.e. baryons=by triplet of quarks/antiquarks & mesons=by pair of quark-antiquark). W+, W-, and Z bosons=carry weak force which controls decay (i.e. way unstable particles change into stable particles) of unstable particles. Graviton=carry gravitational force which is the attraction between any mass-bearing objects. Fundamental force= that govern the interaction between particles and energy in the universe Gravitational force is the weakest-on the scale of elementary particles and atoms.
- 52. FUNDAMENTAL BOSON-PHOTON (has spin=1, no mass or electric charge but carry very high momentum) Very high momentum of photon is because of its high speed equal to speed of light, So when same charged particles approaches to each other, one particle emits this photon & other particle absorbs that photon, thereby repelling each other. I.E. Photon transfers electromagnetic force between particles. FUNDAMENTAL BOSON-GLUON (has spin=1, no mass or electric charge but carry color charge) Color charge has 3 color values (i.e. red, green, blue) & 3 anticolor values (i.e. cyan, magenta, yellow) Combination of all three possible color charges creates a particle with no color charge. Also, combination of a color and its anticolor is colorless. Gravitational force is the weakest-on the scale of elementary particles and atoms.
- 53. FUNDAMENTAL BOSON-W+, W- & Z (has spin=1, no color charge but carry electric charges & masses) Gravitational force is the weakest-on the scale of elementary particles and atoms. W+ and W- bosons have electric charges +1 & –1 respectively, But Z boson has no electric charge. W+ and W- bosons have masses 80 GeV/c2 , And Z boson has mass 91 GeV/c2 . How W+,W- & Z Boson work: A decaying particle changes form and emits one of the W+,W- & Z bosons. The emitted weak boson then decays into other particles. E.G. In positron decay, a quark (in proton) emits W+ boson & converts to other quark. Thus that proton converts into neutron. And the emitted W+ boson immediately decays into a positron & electron neutrino.
- 54. FUNDAMENTAL BOSON-GRAVITON (but not yet detected) (If it exists, will have spin=2 & no mass or electric charge) Gravitational force is the weakest-on the scale of elementary particles and atoms. W+ and W- bosons have electric charges +1 & –1 respectively, But Z boson has no electric charge. W+ and W- bosons have masses 80 GeV/c2 , And Z boson has mass 91 GeV/c2 . How W+,W- & Z Boson work: A decaying particle changes form and emits one of the W+,W- & Z bosons. The emitted weak boson then decays into other particles. E.G. In positron decay, a quark (in proton) emits W+ boson & converts to other quark. Thus that proton converts into neutron. And the emitted W+ boson immediately decays into a positron & electron neutrino.
- 55. COMPOSITE BOSON (i.e. MESON) (It contains even number of elementary/fundamental fermions, i.e. a pair of quark-antiquark) Gravitational force is the weakest-on the scale of elementary particles and atoms. There are six quarks and six antiquarks, so 36 possible mesons may exist. Important mesons are pion, kaon, B, D, F particle. They are formed by 2nd & 3rd Gen. quarks; They are not found in atomic nucleus (rather found during cosmic particle-Earth’s atmospheric atoms interaction) & are Unstable.
- 56. Glow/Luminescence (emission of low-energy light) Vs Incandescence (emission of light by heated materials) Glow (i.e. low-energy light emission at lower temperature than is needed for combustion ) Fluorescence (e.g. Interior of fluorescent lamp, TV screen coated with Phosphor material) Interval between absorption & emission of light Is short (<10-5 second) Phosphorescence Interval between absorption & emission of light Is long (many hours)
- 57. Glow/Luminescence (emission of low-energy light) Vs Incandescence (emission of light by heated materials) Various types of Glow= 1. Chemiluminescence-Oxidization of yellow phosphorus in Air, giving green glow. 2. Bioluminescence-Oxidization of yellow phosphorus in Living organism (e.g. firefly), giving green glow. 3. Triboluminescence-During breaking, pulling, or scratching certain materials. 4. Electroluminescence-By using Gas in the path of electric discharge, e.g. lightning, light of fluorescent lamp 5. Photoluminescence-By using visible or ultraviolet light on certain materials, e.g. phosphorescence of paints 6. Sonoluminescence-By throwing ultrasound in some organic liquids. 7. Roentgenoluminescence-By using X ray on certain materials, e.g. using X ray on fluoroscope screen
- 58. ATOMIC STRUCTURE (proton’s mass is about 1,840 times the mass of an electron, Neutrons are about the same size as protons but their mass is slightly greater.)
- 59. SUBATOMIC STRUCTURE HADRON-composite particle (made by various quarks combination with gluon) BARYON/COMPOSITE FERMIONS -has odd number of quarks (=proton, neutron) MESON/BOSONIC COMPOSITES -has even number of quarks i.e:1 quark-1 antiquark pair (=pion, kaon, B, D, F particle)
- 60. NUCLEAR STRUCTURE The story began about 20 years ago, when Maria Goeppert Mayer at the University of Chicago and O. P. L. Haxel, J. Hans D. Jensen and Hans E. Suess of the University of Heidelberg began to develop a 'shell' model of the nucleus that consisted of particles moving in a field of nuclear force. The collection of particles (neutrons and protons) was shown to be particularly stable when the nucleus contained a 'magic' number of neutrons or protons. The stable structure could be regarded as shells, or spherical orbits, whose capacity for nuclear particles is filled; it is analogous to the filled electron shells of the noble gases. Magic numbers of neutrons (N) or protons (Z) are generally recognized as being 2, 8, 20, 28, 50 and 82 in the elements below uranium in the periodic table. The magic number N = 126 is also significant in this region, as can be seen in the special stability of lead 208 (20882Pb, or Z = 82 and N = 126), which has a doubly magic nucleus. The shell theory has evolved through many stages, to the point where the potential of single nucleons (protons or neutrons) in a deformed (nonspherical) nuclear field can be calculated by using 'Nilsson orbitals,' a method developed by the Swedish physicist Sven Gösta Nilsson. charged liquid-drop model of the nucleus first put forward by Niels Bohr and John A. Wheeler. This model assumes for a stable nucleus a spherical, or liquid-drop, form brought about by a balance between the inwardly directed force of surface tension and the outwardly directed force of repulsion among the positively charged protons. The model has been developed further to help in predicting the stability of heavy nuclei with respect to spontaneous fission and other modes of decay. extension of the liquid-drop model formulated by W. D. Myers and W. J. Swiatecki of the Lawrence Radiation Laboratory the potential energy of the nucleus is a function of neutron number, atomic number and nuclear shape. The nuclei of most isotopes, including those of the lanthanide and actinide elements, have somewhat deformed shapes, for example ellipsoids, but the nuclei of isotopes whose shells are filled or nearly filled are spherical. In the absence of filled shells all superheavy elements would decay instantaneously by spontaneous fission to isotopes with medium atomic numbers located near the middle of the periodic table.
- 61. NUCLEAR STRUCTURE The story began about 20 years ago, when Maria Goeppert Mayer at the University of Chicago and O. P. L. Haxel, J. Hans D. Jensen and Hans E. Suess of the University of Heidelberg began to develop a 'shell' model of the nucleus that consisted of particles moving in a field of nuclear force. The collection of particles (neutrons and protons) was shown to be particularly stable when the nucleus contained a 'magic' number of neutrons or protons. The stable structure could be regarded as shells, or spherical orbits, whose capacity for nuclear particles is filled; it is analogous to the filled electron shells of the noble gases. Magic numbers of neutrons (N) or protons (Z) are generally recognized as being 2, 8, 20, 28, 50 and 82 in the elements below uranium in the periodic table. The magic number N = 126 is also significant in this region, as can be seen in the special stability of lead 208 (20882Pb, or Z = 82 and N = 126), which has a doubly magic nucleus. The shell theory has evolved through many stages, to the point where the potential of single nucleons (protons or neutrons) in a deformed (nonspherical) nuclear field can be calculated by using 'Nilsson orbitals,' a method developed by the Swedish physicist Sven Gösta Nilsson. charged liquid-drop model of the nucleus first put forward by Niels Bohr and John A. Wheeler. This model assumes for a stable nucleus a spherical, or liquid-drop, form brought about by a balance between the inwardly directed force of surface tension and the outwardly directed force of repulsion among the positively charged protons. The model has been developed further to help in predicting the stability of heavy nuclei with respect to spontaneous fission and other modes of decay. extension of the liquid-drop model formulated by W. D. Myers and W. J. Swiatecki of the Lawrence Radiation Laboratory the potential energy of the nucleus is a function of neutron number, atomic number and nuclear shape. The nuclei of most isotopes, including those of the lanthanide and actinide elements, have somewhat deformed shapes, for example ellipsoids, but the nuclei of isotopes whose shells are filled or nearly filled are spherical. In the absence of filled shells all superheavy elements would decay instantaneously by spontaneous fission to isotopes with medium atomic numbers located near the middle of the periodic table.
- 62. MAJOR ATOMIC MODELS • Dalton model=in 1803, depicted atom as tiny, indestructible particle with no internal structure. • Thomson model= in 1898, discovered electron & described that electrons are embedded in positively charged sphere. • Rutherford Model=in 1909, showed most of an atom’s mass is concentrated in centrally & positively charged nucleus. • Bohr model=in 1913, proposed that electrons having definite energies, move in various, circular orbits around nucleus & an atom emits electromagnetic radiation only when an electron in the atom jumps from one quantum level to another. • Wave model=from1926 to present time, stated that electrons move around nucleus as definite quantized waves or energy levels. Existing region(s) of space in each energy level is also quantized accordingly . radius(n) = n2 a0 a0 = Bohr radius = 0.529 Å
- 63. CRITICISM OF MODELS Indivisible Electron Nucleus Orbit Electron Cloud Greek √ Dalton √ Thomson √ Had no idea Rutherford √ √ Had no idea Bohr √ √ √ Wave/Vibra tion (i.e. regular repetitions) √ √ √
- 64. 4 Quantum numbers for Fermions-e- /p+ /n (to define each fermion’s energy in orbitals) Principal (n) QN=specific energy level, Secondary/Angular (l) QN=number of orbital, Third/Magnetic (m) QN=number of direction of orbital Fourth/Spin (i.e. intrinsic angular momentum) QN (ms)=number of direction of rotation Pauli exclusion principle=2 identical fermions (not bosons) cannot occupy same quantum state/orbital. (i.e. describe arrangement & behavior of fermions in orbitals) [fermions have spin of odd half integers e.g. ½,3/2, 5/2 etc and bosons have spin of whole integers e.g. 0,1,2 etc.] ORBITAL=pattern of rotation around nucleus by specific amount of energy-bearing rotating electron. In 1927, Paul Dirac theorized that e- has spin & antiparticle In 1925, Pauli developed exclusion principle, In 1926, Enrico Fermi set rules that define behavior of fermions In late 1926, e- is regarded as fermion
- 65. 4 Quantum numbers for Fermions-e- /p+ /n (to define each fermion’s energy in orbitals) Principal (n) QN=specific energy level, Secondary/Angular (l) QN=number of orbital, Third/Magnetic (m) QN=number of direction of orbital Fourth/Spin QN (ms)=number of direction of rotation no two electrons in an atom can have identical sets of quantum numbers= Exclusion principle of Austrian-Swiss Physicist W. Pauli.
- 66. 4 Quantum numbers for Fermions-e- /p+ /n (to define each fermion’s energy in energy levels & orbitals) Principal (n) QN=specific energy level, Secondary/Angular (l) QN=number of orbital, Third/Magnetic (m) QN=number of direction of orbital Fourth/Spin QN (ms)=number of direction of rotation Arrangement of electrons in an atom depends on: 1. Quantization of electron energy, which limits the regions of space that electrons can occupy 2. Pauli exclusion principle (i.e. identical fermions cannot occupy same quantum state/orbital) n=1,2,3 etc l=0 to n-1 (1n=l=0=s, 2n=l=0&1=s&p, 3n=l=0,1&2=s,p&d, 4n=l=0,1,2&3=s,p,d&f orbital) m=2l+1 (l=0=s=1, l=1=p=3, l=2=d=5, l=3=f=7) 2n2 (thus 2,8, 18, 32 in n=1,2,3,4 respectively) 2(2l+1) (thus 1n=l=0=s=2, 2n=l=0&1=s&p=2&6 3n=l=0,1&2=s,p&d=2,6&10 4n=l=0,1,2&3=s,p,d&f=2,6,10&14 ) Max. no. of electrons Color charge enables two up or down quarks in protons and neutrons to be different from each other, thereby exclusion principle is not violated by the Quark fermions.
- 67. ATOMIC ENERGY LEVELS ELECTRON ENERGY LEVEL= • Every element has definite energy levels i.e. definite energy transitions i.e. definite frequencies and wavelengths of light which can be emitted the element (solid, liquid or gas) is given enough energy via heating or electric current. They give rise to definite emission (continuous or line) spectrum. By seeing line spectrum, an element can be identified definitely. • Energy levels referred to the orbitals/regions of space in atoms where electrons with same energies may present with 90% chance. • The level closest to nucleus, has electrons with lowest energy & the level farthest from nucleus, has electrons with highest energy. • In a stable atom, electrons firstly fill the lowest energy levels and consistent with the pauli exclusion principle, the aufbau principle, and hund's rule. • Photon is emitted when electrons move from high energy level to low energy level. And the energy of photon will be equal to difference between these levels
- 68. ATOMIC ENERGY LEVELS GROUND/STABLE STATE of atoms= Complete & orderly filling up of innermost energy levels, closest to nucleus by electrons indicate this state. Atom’s energy is minimum at this state. EXCITED STATE of atoms= Very transient (10-13 ,10-14 … SECOND) presence of electrons in outer energy level in excited atoms indicate this state. When atoms are excited by heat, electricity, light or some other form of radiation, atoms’ electrons instantly absorb energy & jump from inner to outer energy level for transient period and then emit equal amount of absorbed energy (as photon/tiny flash of light energy) to fall back to inner, vacant energy level. Each excited elements’ atom’ electronic structure thus shows a distinct set of emitted energies (i.e. colors of light)-studied by spectroscopy. 3.METASTABLE STATE/ISOMERIC STATE= RELATIVELY LONG-LIVED (second, minute or even year; 10-12 SECOND or more.) EXISTENCE OF EXCITED STATE ELECTRONS OR NUCLEONS IN LOWER ENERGY LEVEL. SPONTANEOUS ENERGY EMISSION IS OCCURRED DURING THE CONVERSION OF EXCITED STATE TO METASTABLE STATE & THEN TO GROUND OR LOWER ENERGY STATE ELECTRONS OR NUCLEONS.
- 69. ATOMIC ENERGY LEVELS EXCITED STATE METASTABLE STATE GROUND STATE APPLICATIONOFENERGY (FORIT’SINDUCED ABSORPTIONINTO ELECTRONSOR NUCLEONS) SPONTANEOUS EMISSION OF ENERGY STIMULATED EMISSION OF RADIATION
- 70. EXCITATION & IONIZATION OF ATOM 1. EXCITATION=PROCESS OF RAISING LOWER ENERGY LEVEL ORBITAL ELECTRONS OR NUCLEONS TO UNOCCUPIED HIGH ENERGY LEVEL SHELLS OR ORBITS. 2. IONIZATION= PROCESS OF CREATING CHARGED ATOM. HERE ELECTRON IS REMOVED FROM A ATOM. THUS AN ION PAIR IS PRODUCED. HOW THEY CAN BE PRODUCED= BY 1. CHARGED PARTICLE-MATTER INTERACTION 2. Heat 3. Electricity 4. Light Atoms are made excited by heat, electricity, light or other form of radiation.
- 71. RADIATION Radiation=Emitted energy from unstable atom, which is particulate or wave in nature. Ionizing radiation=High energy radiation, which is capable of removing
- 72. ACTIVITY & RADIOACTIVITY OF A RADIOACTIVE SAMPLE Activity=number of nuclei decaying per second. Measured in becquerels (Bq) in S.I unit or curie (Ci) conventional unit. (1 Ci=3.7 x 1010 Bq) Radioactivity=spontaneous disintegration or decaying of radionuclide with emission of radiation. Radioactive transformation (or, decay) =various spontaneous ways through which unstable nuclide loses its energy.
- 73. NUCLIDE NUCLIDE=atomic nucleus which is characterized by its nucleon numbers & energy content. Varieties- (An element’s identity is determined its proton number) Isotopes-nuclides of a same element having same proton number (Z) but different neutron numbers (N) [hence different mass numbers (A)]. EXAMPLE=C-14(unstable), C-12 (stable); Isobars-nuclides of different elements having same mass number (A) but different proton (Z) & neutron (N) numbers. EXAMPLE=38Sr-90 (unstable)- 39Y-90 (unstable)-40Zr-90 (stable) Isotones-nuclides of different elements having same neutron number (N) but different mass (Z) & proton numbers (A). Isomers-excited or metastable state of nuclides (i.e. unstable or radionuclides) of a same element. A transition from one isomer to another is accompanied by emission or absorption of a gamma ray, or the process of internal conversion NUCLIDE NATURAL 280 (stable)+ 83 (unstable) ARTIFICIAL >3000
- 74. CHART OF NUCLIDES Stable Nuclides
- 75. NUCLIDE STABILITY & RADIOACTIVE/DECAY SERIES/SCHEME/CHAIN From viewpoint of forces- Balance between two forces make nuclide stable • Strong (nuclear) force- hold nucleons together. • Electromagnetic force-hold protons together. From viewpoint of neutron (N) & proton (Z) number- • N=Z, for lighter nuclide (A<50). • N>Z, for heavier nuclide (A>100). Radioactive/Decay series=when a radionuclide decay into stable nuclide via other radionuclides. example- 99 Mo-99m Tc-99 Tc-99 Ru (stable)
- 76. FORCES/WAVES/ENERGYS/INTERACTIONS (They define everything’s nature & interaction among everything; These waves are massless & travel at the speed of light; Fundamental particle Bosons create these 4 forces.) This attractive force acts between every mass-carrying Objects. Quantum/Discrete packet of EM energy is called photon This nuclear force binds protons & neutrons in atom’s nucleus. It acts on every electric charge(+/-)-carrying Objects. Quantum/Discrete packet of GRV energy is called graviton Quantum/Discrete packet of STR energy is called gluon Quantum/Discrete packet of WK energy is called W,Z
- 77. RADIOACTIVE DECAY (mediated by weak force/interaction which is carried by W & Z bosons) Beta decay (Isobaric transition)-Here nucleons are into opposite particles. Energy = few KeV to 10 MeV. 2 types (Beta particles include high energy electron & positron ejected from radionuclide.) 1.Electron emission/Beta minus/Negatron (in radionuclides having excess neutrons)=Here proton, electron (e- ) & anti-electron neutrino are released from a neutron. Example:90 Sr38=e- +90 Y39=e- +90 Zr40 (stable), 99 Mo42 =e- +99m Tc43=e- +99 Ru44 (stable) 2.Positron emission/Beta plus (in low atomic weight radionuclides having excess protons)=Here neutron, positron (e+ ) & electron neutrino are released from a proton. Then positron annihilates with orbital electron & produce high energy (25 Mev) gamma radiation. Example:18 F9=e+ +18 O8 3.Electron capture (EC) (in higher atomic weight radionuclides having excess protons)=Here neutron & neutrino are released from a proton when it absorbs inner orbital electron. Here simultaneous emission of characteristic x-ray or Auger electron occurs. Example:125 I53=125m Te52(metastable state) “Alpha or beta emission usually leaves the nucleus with excess energy, which it unloads by emitting a gamma-ray photon.”
- 78. RADIOACTIVE DECAY (mediated by weak force/interaction which is carried by W & Z bosons) Alpha decay [in radionuclide of very heavy element having excess neutrons like radium (Ra), thorium (Th), uranium (U)-Here Alpha particle is ejected from]=Here low speed, heavy Alpha particle having 2 protons & 2 neutrons is released. It is identical to helium nucleus and has 2 positive charge. Energy = 3 to 7 MeV. Example:226 Ra88=α2+ +222 Rn86gas (stable) Gamma decay [in radionuclide of very heavy element having excess neutrons like radium (Ra), thorium (Th), uranium (U)-Here Alpha particle is ejected from]=Here low speed, heavy Alpha particle having 2 protons & 2 neutrons is released. It is identical to helium nucleus and has 2 positive charge. Energy = 3 to 7 MeV. Example:226 Ra88=alpfa2+ +222 Rn86gas (stable) Internal conversion (IC) decay (emission of characteristic x-ray or Auger electron in radionuclide of same element )=Here inner orbital electron is emitted by the kinetic energy which comes from nuclear transition from high energy level to lower energy level. Here simultaneous emission of characteristic x-ray or Auger electron occurs. But no gamma ray emission is seen. Example:125m Te52=125 Te52(stable)
- 79. ACCELERATORS (in 1932=cockcroft,walton,van de graaf for generator & lawrence,livingston for cyclotron & mcmillan for synchroton) Energize/accelerate voltage of beams of electrons, protons, deuterons, heavier ions, and X rays to probe atom High energy type used in research on elementary particles & low energy type used is applications inside industries and laboratories “Alpha or beta emission usually leaves the nucleus with excess energy, which it unloads by emitting a gamma-ray photon.”
- 80. IONIZATION ENERGY & NUCLEAR BINDING ENERGY 1. IONIZATION ENERGY/ORBITAL ELECTRON BINDING ENERGY= ENERGY REQUIRED TO REMOVE AN ELECTRON FROM AN ATOM. IT IS MEASURED IN ELECTRON VOLT (eV), THIS ENERGY IS INVERSELY PROPORTIONAL TO ATOMIC SIZE. 2. NUCLEAR BINDING ENERGY= ENERGY REQUIRED TO DISASSEMBLE A NUCLEAS INTO FREE PROTONS AND NEUTRONS. IT IS MEASURED IN MEGA ELECTRON VOLT (MeV). THIS ENERGY IS EXPRESSED BY THE FORMULA- E (NUCLEAR BINDING ENERGY) =m( NUCLEAR MASS) x c (LIGHT VELOCITY)2 **MASS DEFICIT/DEFECT/DEFICIENCY= MASS OF AN ATOM OR NUCLEUS IS LESS THAN THE SUM OF MASSES OF ITS INDIVIDUAL CONSTITUENTS. IT IS CONVERTED INTO BINDING ENERGY ACCORDING TO EINSTEIN’S THEORY. IT IS MEASURED IN ENERGY UNIT.
- 81. PHOTO-ELECTRIC EFFECT In 1905, Einstein showed that electrons are ejected upon hitting of metals by lihgt.
- 82. ATOMIC EMISSIONS 1. CHARACTERISTIC X RAY= IT IS AN ELECTROMAGNETIC RADIATION OF ABOUT 100 eV OR MORE ENERGY. IT IS PRODUCED DURING OUTER ORBITAL ELECTRON TRANSIT INTO INNER VACANT ORBIT. 2. AUGER ELECTRON= IT IS AN EMITTED ELECTRON WHICH IS EMITTED FROM THE OUTER ORBITAL AS A RESULT OF ENERGY WHICH IS PRODUCED DURING OUTER ORBITAL ELECTRON TRANSIT TO INNER VACANT ORBITAL. **IN AUGER EFFECT, TWO ELECTRONS ARE REMOVED FROM THE ATOM (DOUBLY CHARGED ATOM). **USUALLY ELEMENTS WITH LOW ATOMIC NUMBER (Z<24) EMIT AUGER ELECTRON & ELEMENTS WITH HIGH ATOMIC NUMBER (Z>45) EMIT X RAY.
- 83. ATOMIC EMISSIONS
- 84. ATOMIC EMISSIONS 1. PAULI’S EXCLUSION PRINCIPLE= TWO ORBITAL ELECTRONS, BEING ON A SAME ORBIT OR SUBSHELL MUST SHOW OPPOSITE SPIN. 2. HUND’S RULE (OF MULTIPLICITY)= IN CASE OF ORBITS OR SUBSHELLS HAVING SAME ENERGY LEVEL, ELECTRONS OCCUPY THOSE ORBITS OR SUBSHELLS SINGLY AND SHOW THE SAME SPIN. 3. AUFBAU BUILDING UP PRINCIPLE= LOW ENERGY LEVEL ORBITS OR SUBSHELLS ARE FILLED UP FIRST BY ORBITAL ELECTRONS THAN THE HIGH ENERGY LEVEL ORBITS OR SUBSHELLS.
