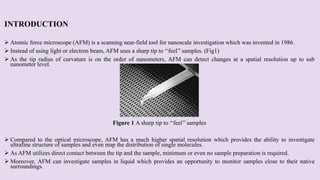
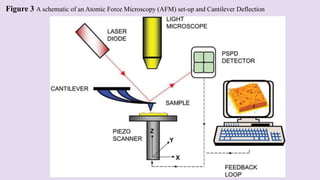
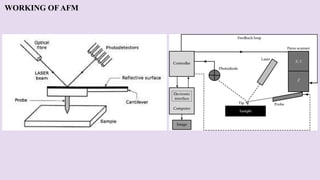
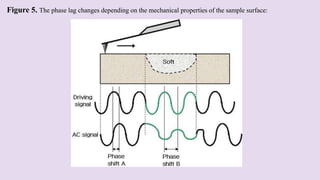
Atomic force microscopy (AFM) is a scanning probe technique capable of imaging surfaces at high resolution. AFM uses a sharp tip that feels the sample surface to detect sub-nanometer level changes. AFM can image samples in liquid and requires minimal sample preparation. The document discusses various combinations of AFM with other techniques like infrared spectroscopy, optical microscopy, and fluorescence microscopy. It also covers the basic principles, working, scanning modes, applications and advantages of AFM for imaging polymers and other materials.