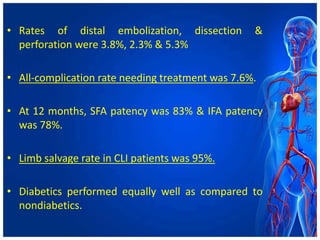
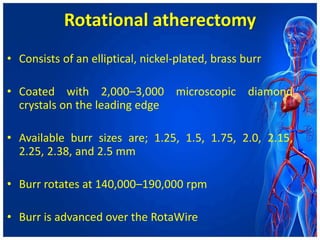
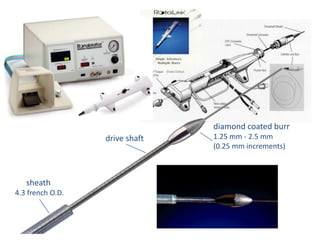
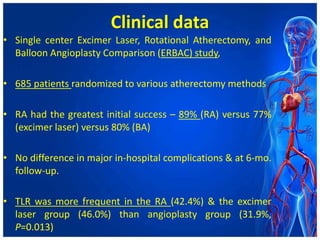
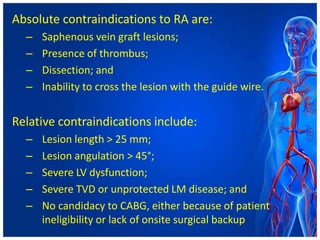
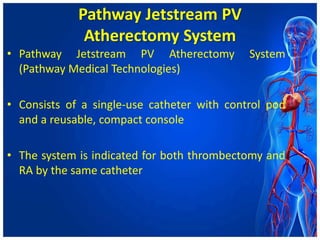
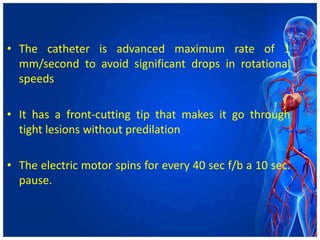
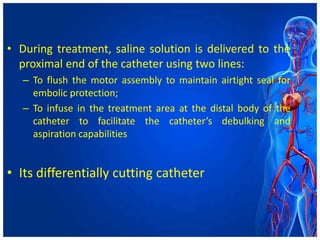
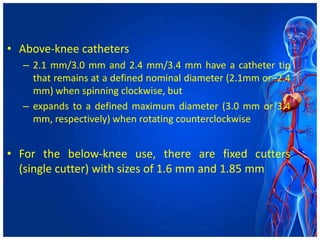
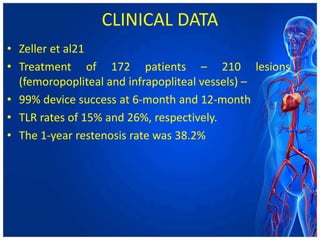
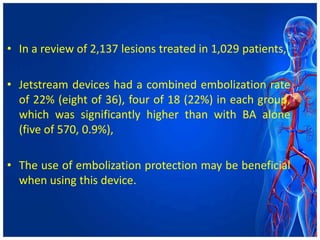
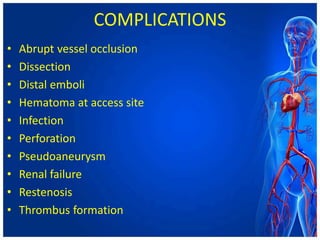
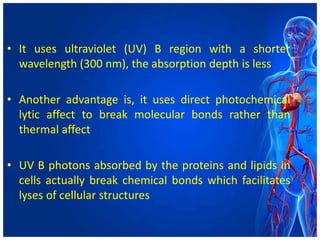
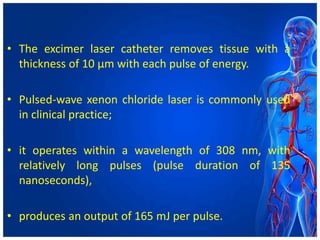
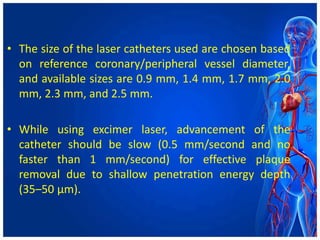
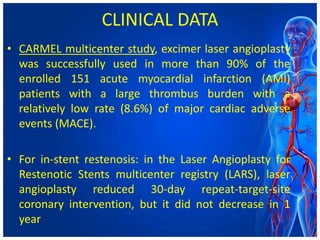
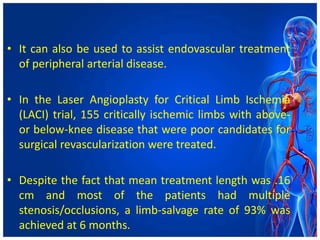
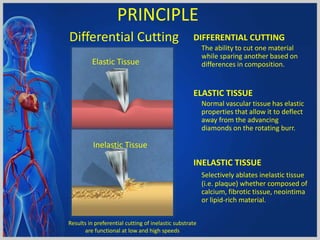
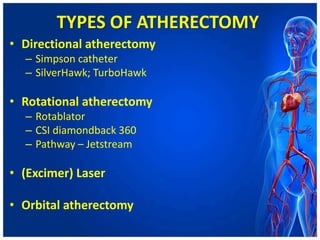
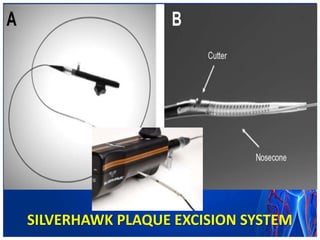
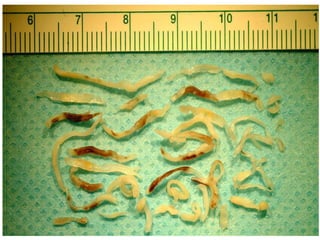
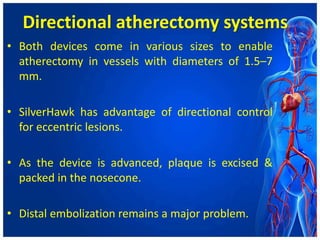
Atherectomy devices aim to reduce plaque in blood vessels using various techniques such as directional, rotational, and laser-based methods, allowing for targeted removal of atherosclerotic material while minimizing damage to surrounding tissues. Clinical studies indicate that while these devices can effectively debulk plaque, their long-term benefits compared to balloon angioplasty alone are not significant, and they come with risks of complications like distal embolization and dissection. Despite these concerns, they remain valuable alternatives for challenging cases where traditional angioplasty may not succeed.








![CLINICAL DATA
• DEFINITIVE LE (Determination of Effectiveness of
SilverHawk Peripheral Plaque Excision [SilverHawk
Device] for the Treatment of Infrainguinal
Vessels/Lower Extremities) registry – largest ever
conducted across 50 sites US & Europe
• Enrolled 799 patients with both claudication and CLI.
• Lesions up to 20 cm in length and multilevel lesions
• Success was reported at 89%, with a post-atherectomy
BA rate of 33% & bail-out stenting rate of 3%.](https://image.slidesharecdn.com/atherectomydevices-210721093739/85/Atherectomy-devices-16-320.jpg)