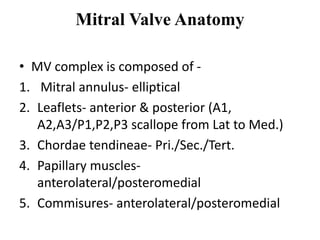
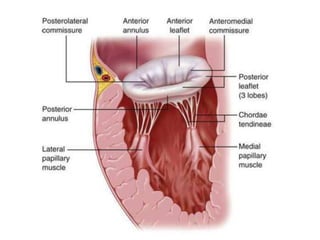
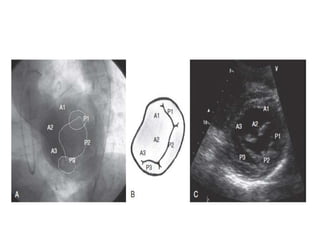
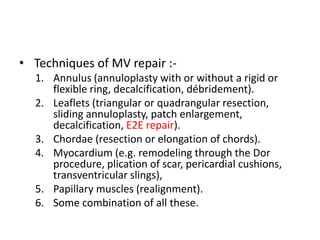
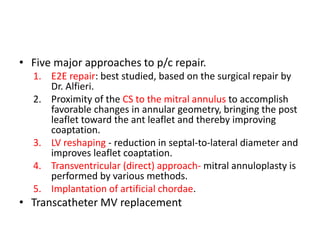
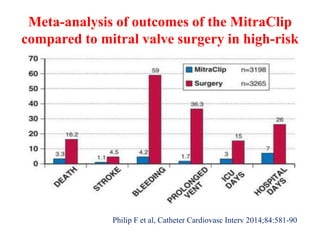
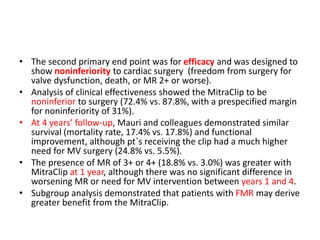
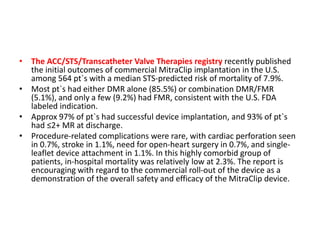
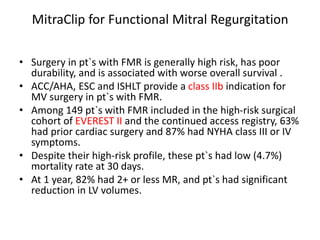
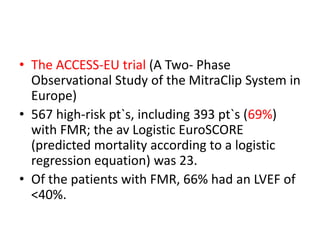
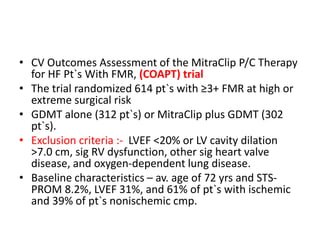
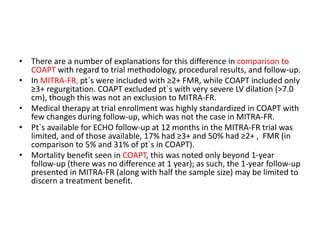
1. The document describes the MitraClip procedure for percutaneous mitral valve repair.
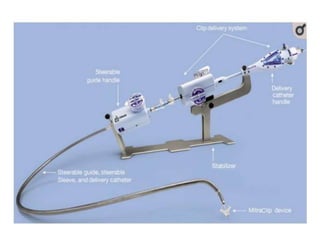
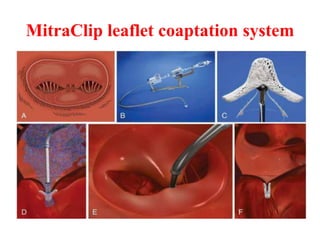
2. The MitraClip procedure involves inserting a clip via the femoral vein to grasp and repair the mitral valve leaflets in a technique similar to the surgical Alfieri repair.
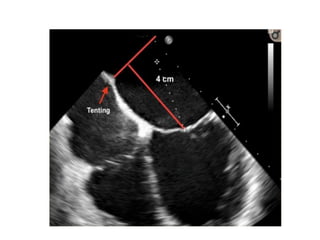
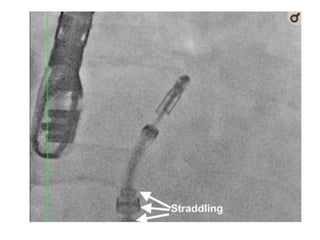
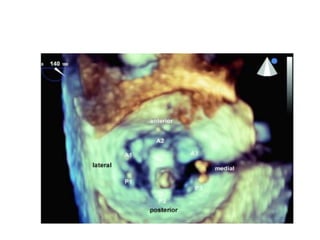
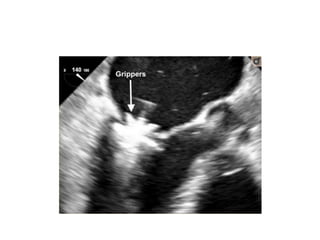
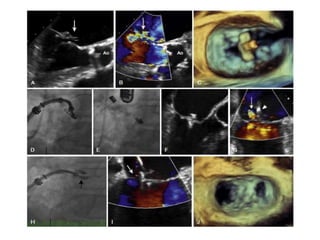
3. The key steps involve transseptal puncture, advancing the clip delivery system into the left atrium, positioning the clip below the mitral valve, grasping the leaflets with the clip, and releasing the clip to create a double orifice mitral valve.