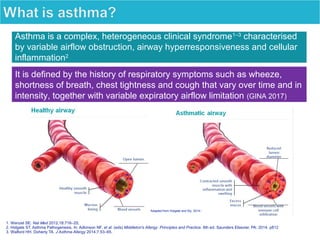
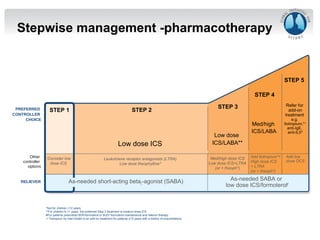
Dr. Yasser Morsy's presentation covers the classification, selection, and treatment of asthma, detailing various medications, their mechanisms of action, usual dosages, and potential side effects. Asthma is described as a complex syndrome characterized by respiratory symptoms and variable airflow obstruction, with management guidelines recommending medications based on the severity of symptoms. The document emphasizes the importance of inhaled corticosteroids and their role in long-term asthma control, along with considerations for combination therapies.






.
Budesonide (Pulmicort Turbohaler® ),
Fluticasone (flixotide Evohaler® 50 , 125 mcg).
Mometasone (Asmanex®).
Ciclesonide (Alvesco®)
Usual doses:
Beginning dose depends on asthma control then titrated down
over 2-3 months to lowest effective dose once control is
achieved.
The British Guideline says 100-400μg daily of Beclometasone
Bipropionate [BDP], or equivalent, is appropriate for most
adults
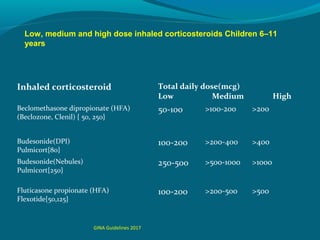
The recommended “standard dose” in children is 100-200
μg/day of [BDP], or equivalent.
13
Leuppi JD, Schuetz P, Bingisser R, et al. Shortterm vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the
REDUCE randomized clinical trial. JAMA 2013;309:2223-31.](https://image.slidesharecdn.com/asthmamedications9-180729062519/85/Asthma-medications-9-13-320.jpg)

![Inhaled corticosteroid Total daily dose(mcg)
Low Medium High
Beclomethasone dipropionate (HFA)
(Beclozone, Clenil) { 50, 250}
100-200 >200-400 >400
Budesonide(DPI)
Pulmicort{80}
200-400 >400-800 >800
Budesonide(Nebules)
Pulmicort{250}
250-500 >500-1000 >1000
Fluticasone propionate (HFA)
Flexotide{50,125}
100-250 >250-500 >500
Combined medications:
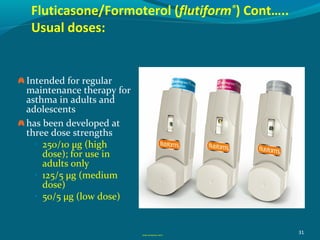
Budesonide and Formetrol ( Symbicort) 160/4.5 , 320/9.
Fluticasone and Salmetrol ( Seretide) 50/25 , 125/25, 250/50 , 500/50
Fluticasone and Formetrol (flutiform®) 250/10, 125/5, 50/5
Low, medium and high dose inhaled corticosteroids Adults and
adolescents (≥12 years)
GINA Guidelines 2017
[HydroFluoroAlkane ]](https://image.slidesharecdn.com/asthmamedications9-180729062519/85/Asthma-medications-9-15-320.jpg)