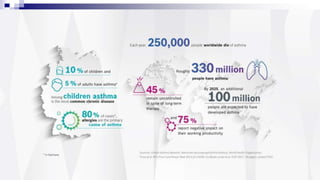
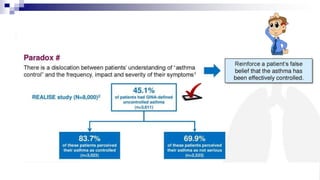
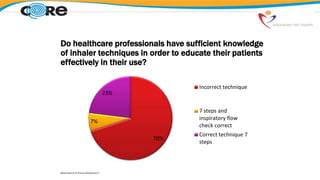
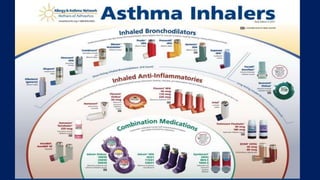
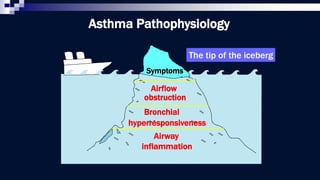
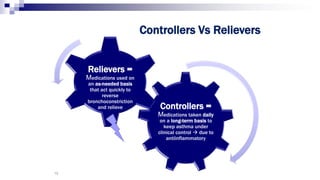
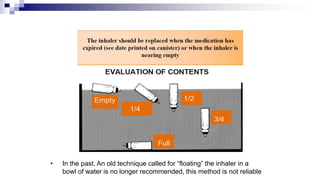
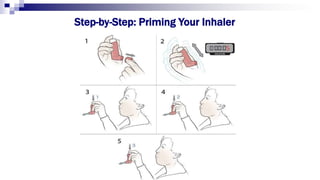
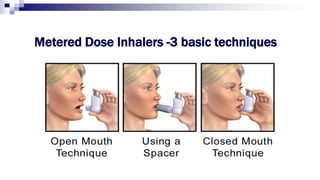
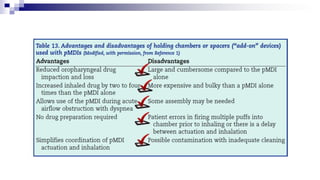
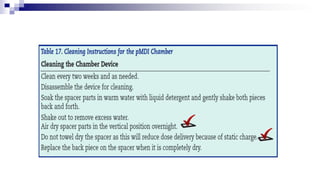
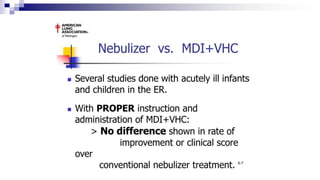
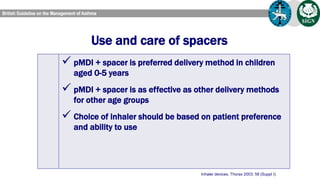
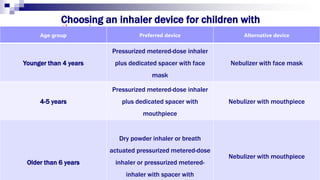
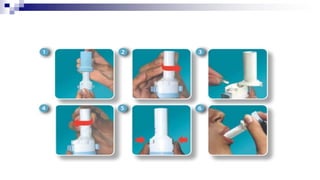
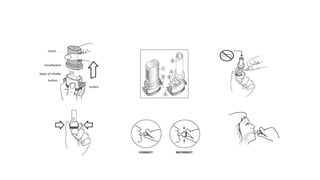
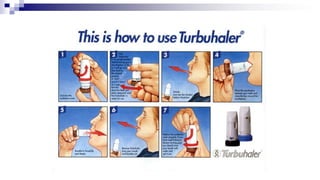
The document discusses reasons for poor asthma control and strategies for improving inhaler technique and medication adherence. Some key points include:
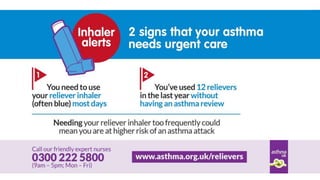
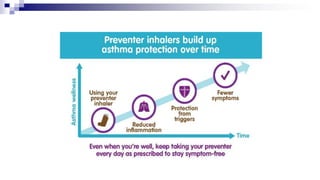
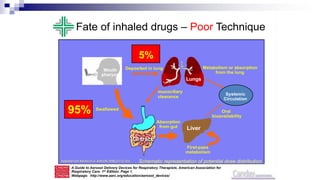
- Poor asthma control can be due to incorrect diagnosis, improper inhaler technique, smoking, comorbid rhinitis, nonadherence to treatment, or inadequate treatment.
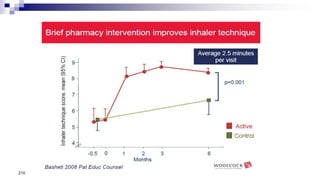
- Healthcare providers need proper training to effectively educate patients on correct inhaler use.
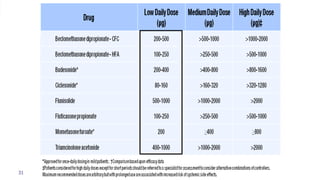
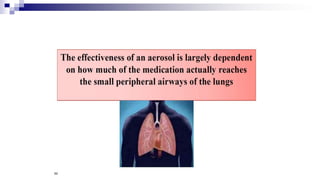
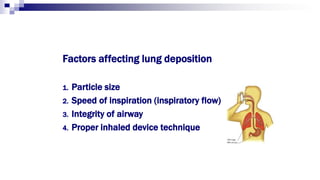
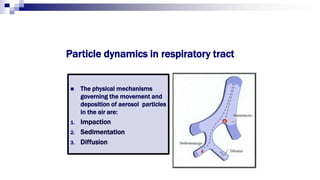
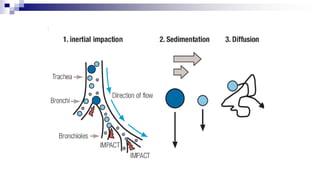
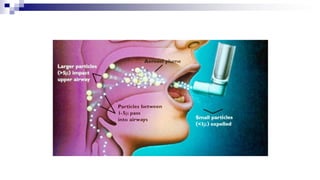
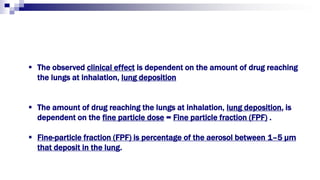
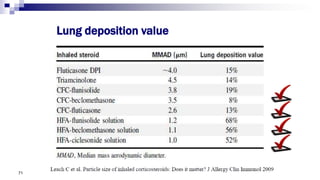
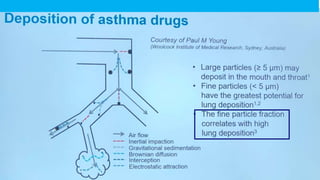
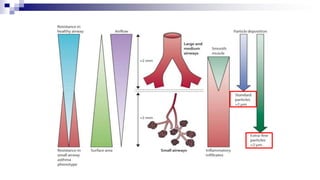
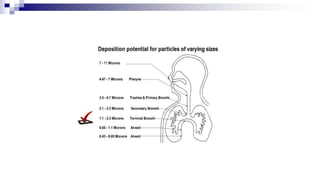
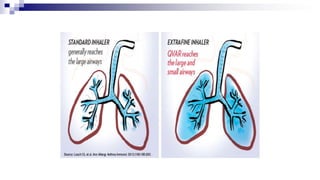
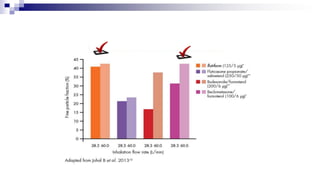
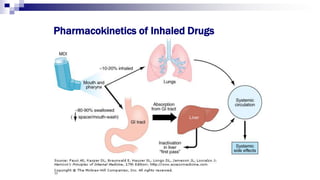
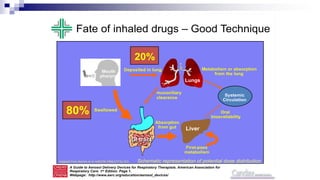
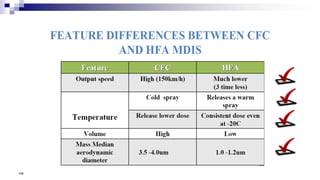
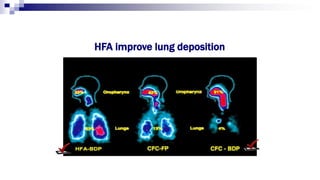
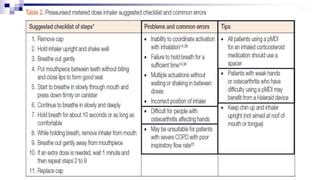
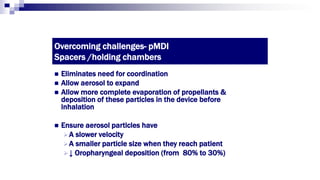
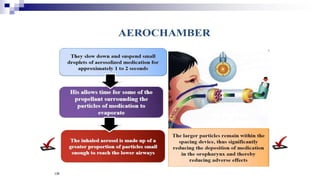
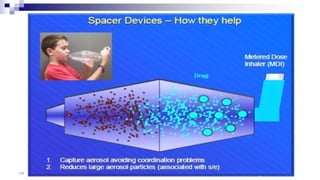
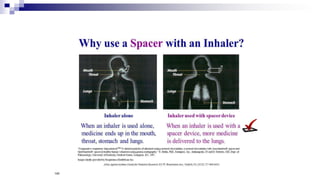
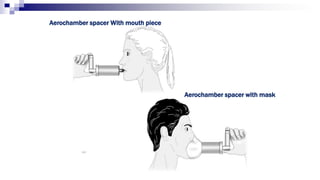
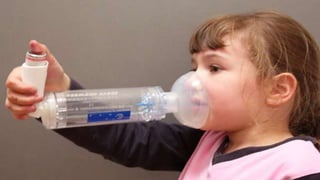
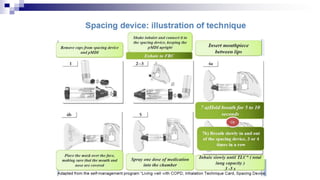
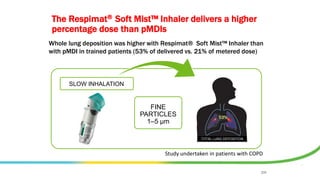
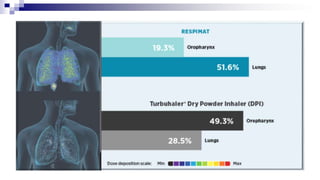
- Factors like particle size, inspiratory flow, and inhaler technique affect lung deposition and treatment effectiveness.
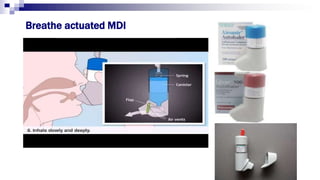
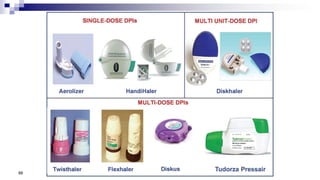
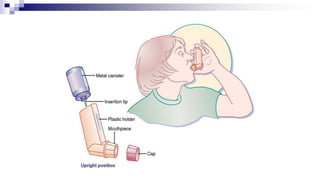
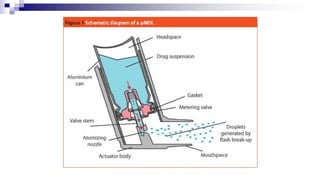
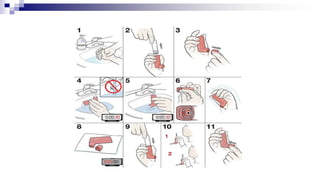
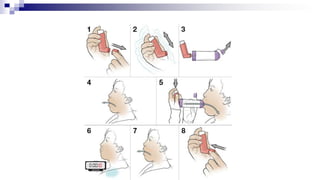
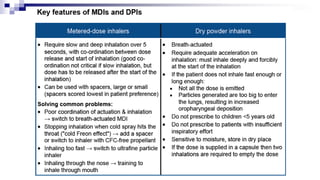
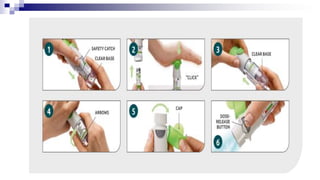
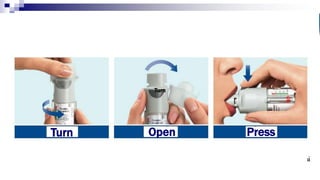
- Common inhaler devices include pressurized metered dose inhalers, dry powder inhalers, and soft mist inhalers. Proper priming, shaking, exhal