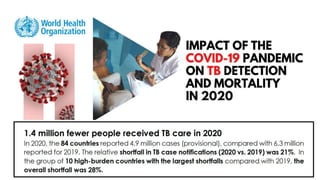
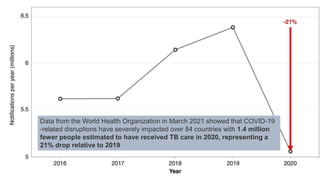
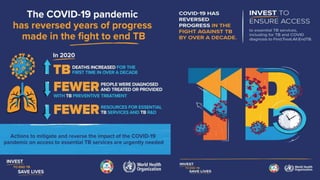
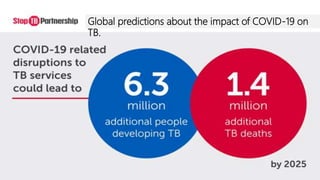
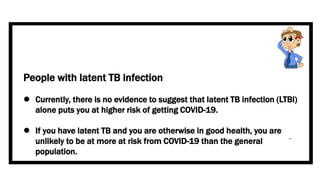
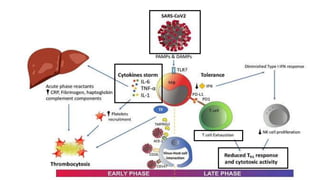
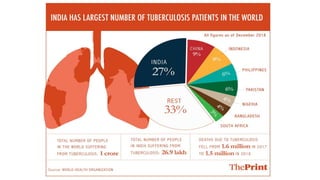
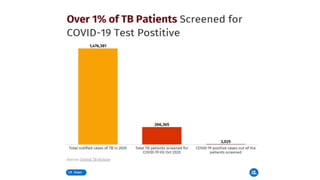
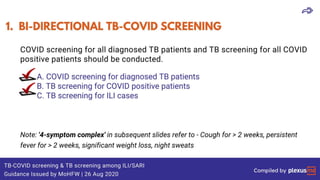
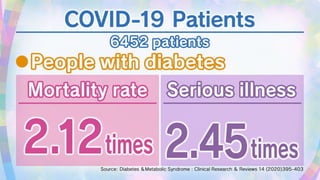
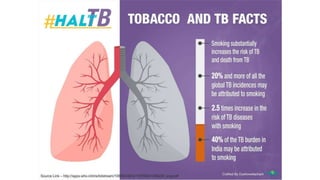
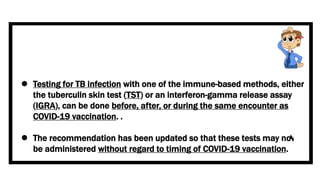
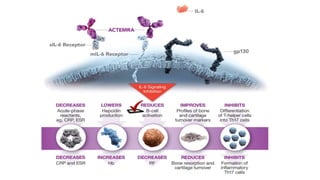
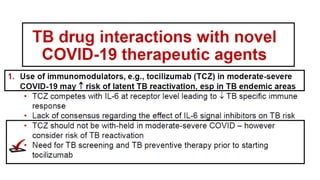
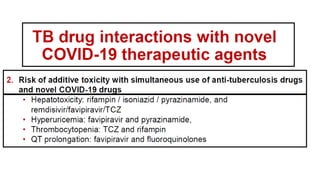
This document discusses the link between COVID-19 and tuberculosis (TB). It notes that COVID-19 disruptions have severely impacted TB treatment and care. It discusses whether TB increases risk for COVID-19 or vice versa, and notes that lung damage from TB may increase COVID-19 risk. The use of corticosteroids for COVID-19 could increase risk of reactivating latent TB infections. Screening for both diseases is recommended. Managing both diseases simultaneously may require continued TB treatment. Vaccines for both are generally safe and should not be delayed. Certain drug interactions between TB and COVID-19 treatments are also discussed.