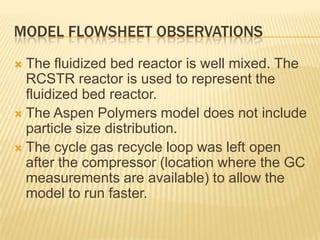
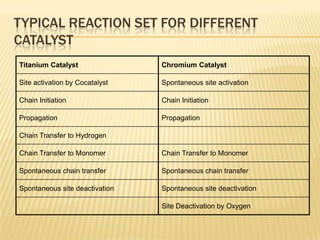
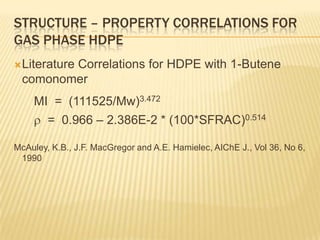
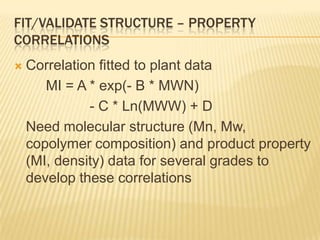
This document provides an overview of the steps taken to implement an Aspen Polymer model of a polyethylene polymerization process. Key steps included developing the process flowsheet, defining components, selecting thermodynamic and kinetic models, inputting operating conditions, and setting up structure-property correlations. The fluidized bed reactor was modeled using a well-mixed reactor block. Component properties were obtained from databases or approximated based on similar molecules. A PCSAFT equation of state was selected to model interactions in the multi-component system operating under pressure. Typical reactions were included for the titanium-based catalyst system.