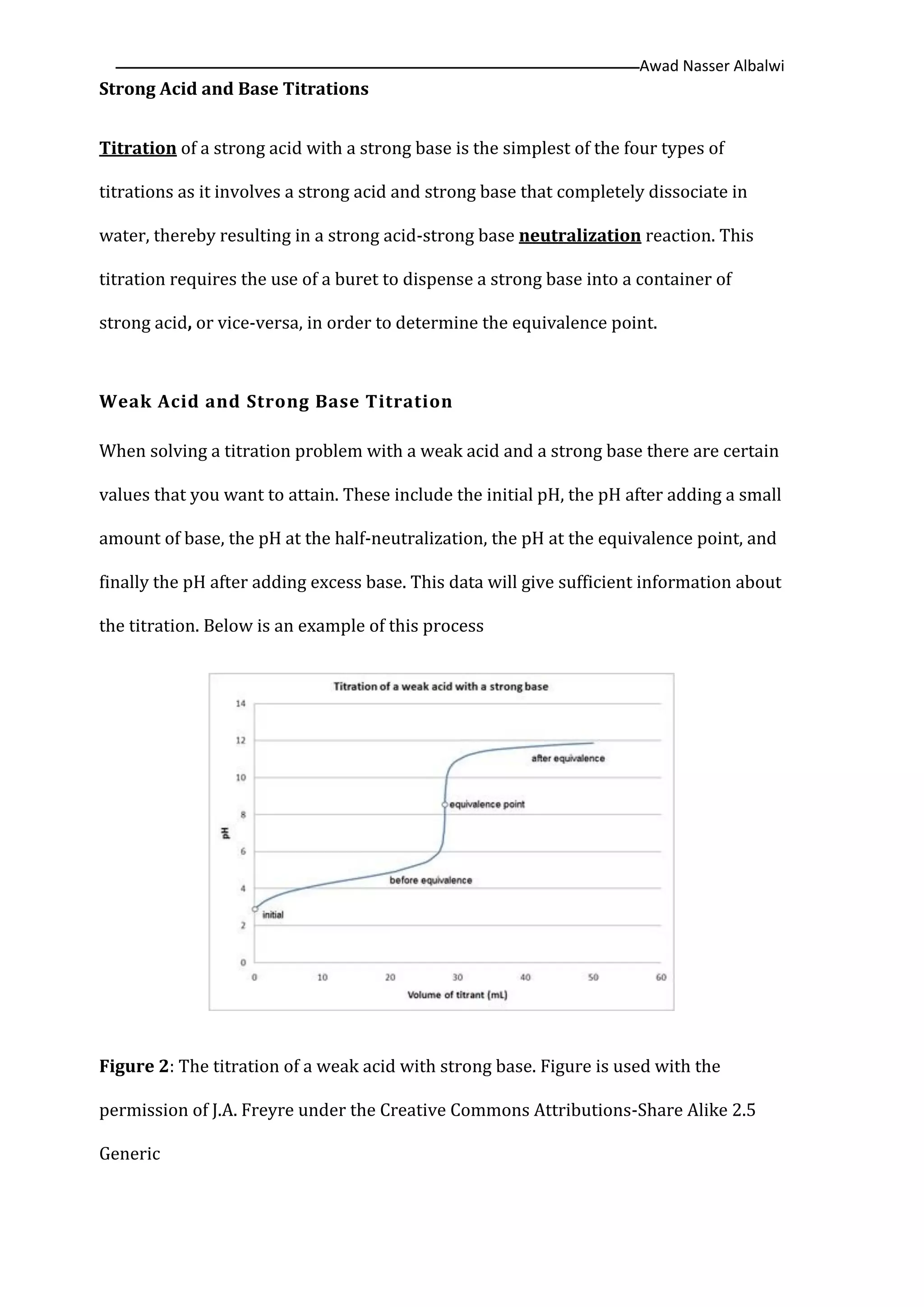
The document discusses various titration methods in chemistry, focusing on strong acid and base titrations, weak acid and strong base titrations, and strong acid and weak base titrations, highlighting their procedures and required calculations for determining pH changes. It also covers precipitation processes, defining precipitation in both liquid and solid states, and introduces gravimetric methods of analysis based on mass measurement. Key concepts include the formation of precipitates, stoichiometric calculations, and gravimetric factors necessary for accurate analysis.