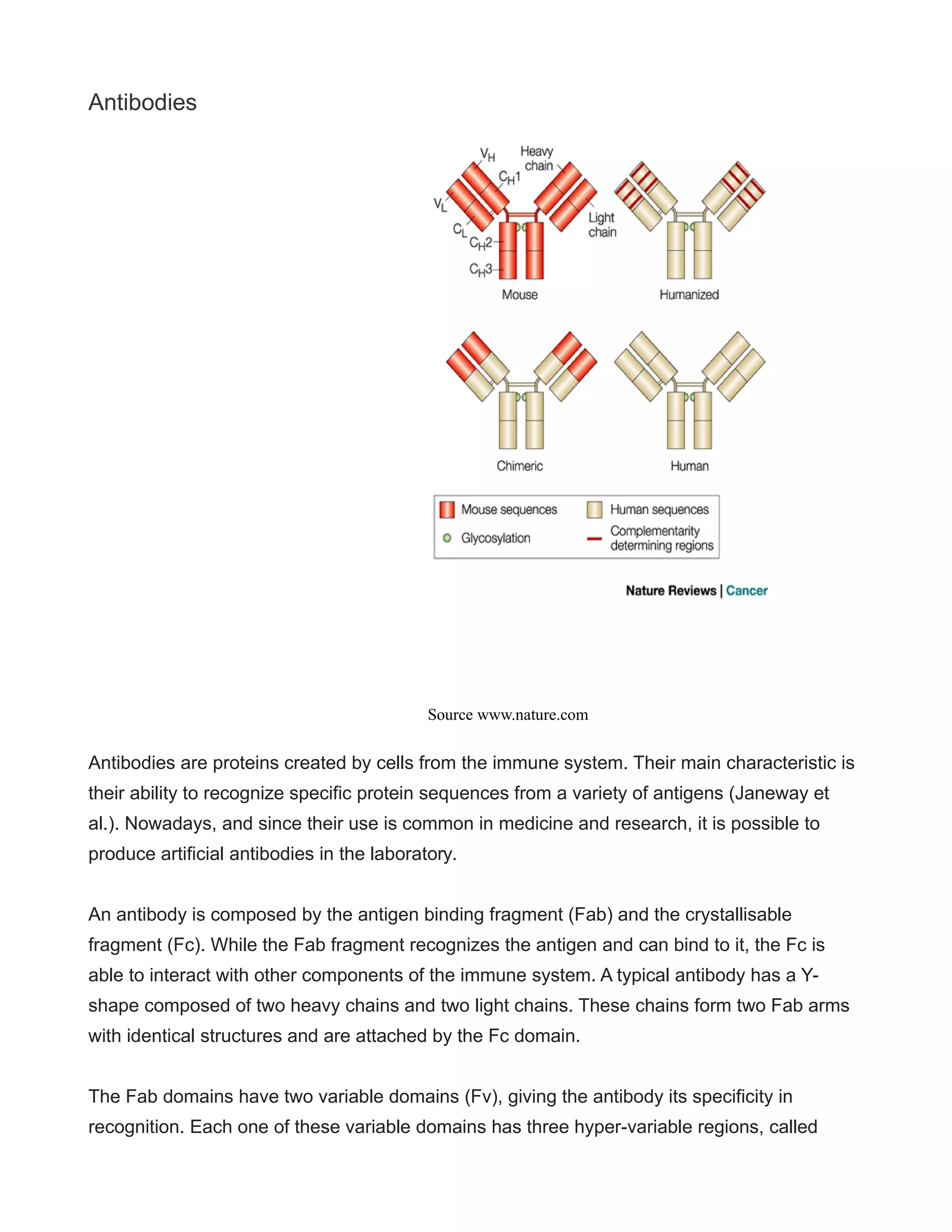
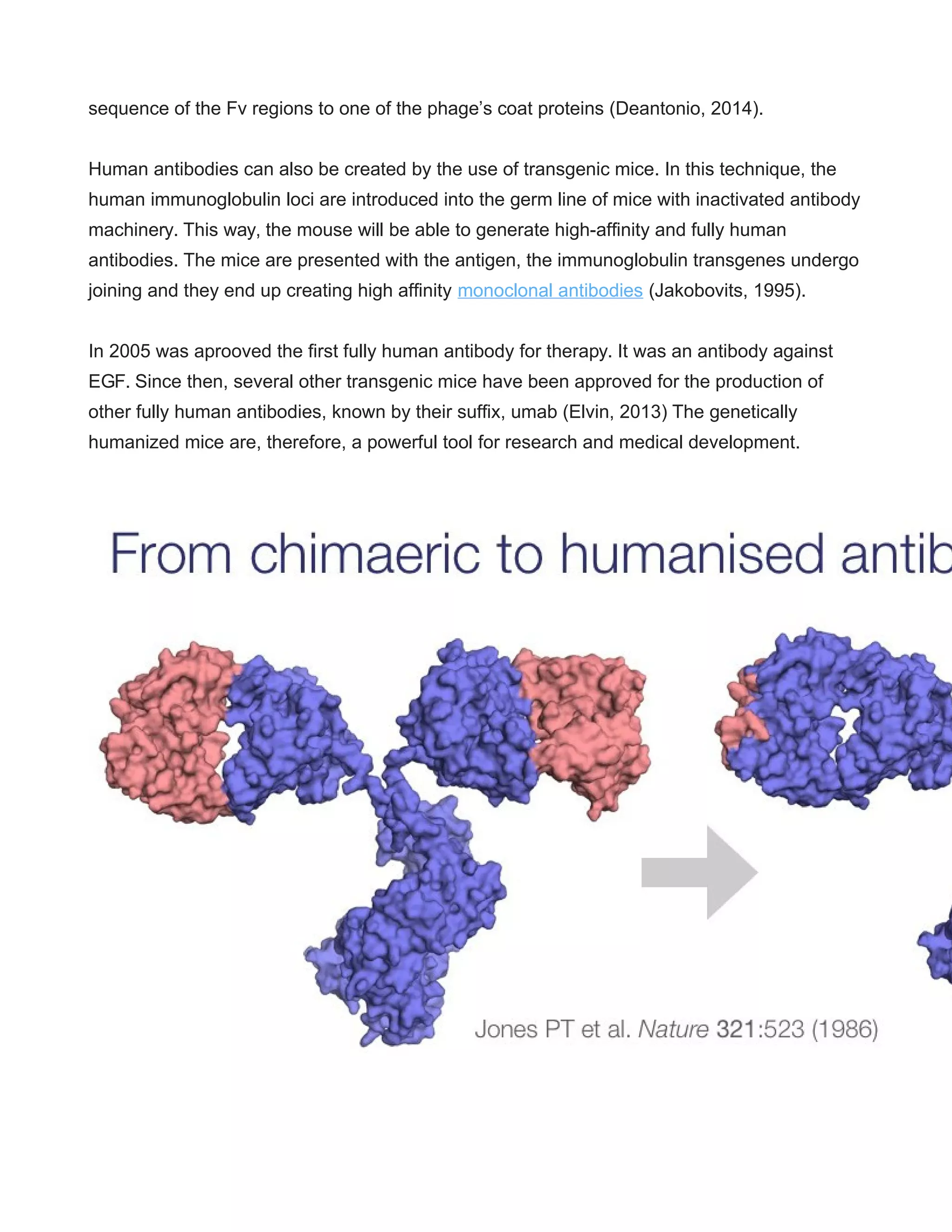
This document provides a comprehensive overview of antibody humanization techniques, which aim to modify antibodies to reduce immunogenic responses when used therapeutically in humans. It discusses various strategies including chimeric and humanized antibodies, emphasizing their importance in treating diseases like cancer and autoimmune disorders. The document also highlights the advancements in generating fully human antibodies and the potential for future therapeutic applications.