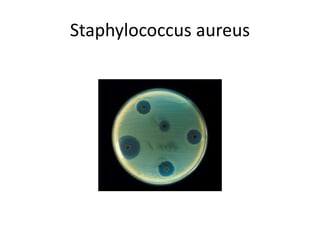
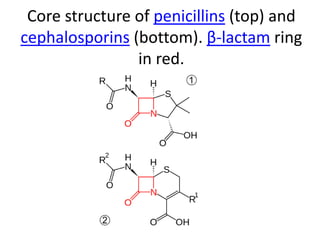
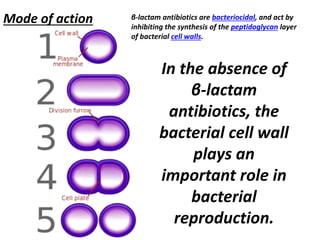
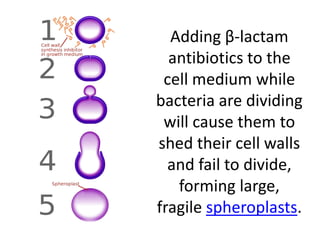
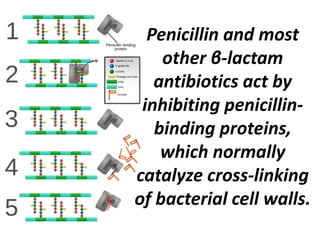
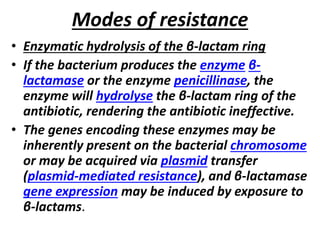
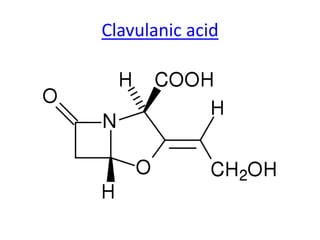
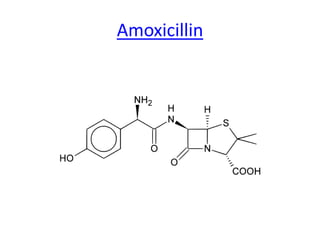
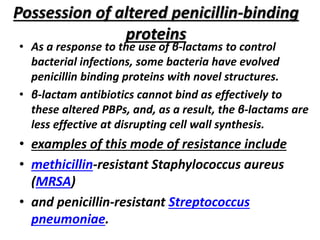
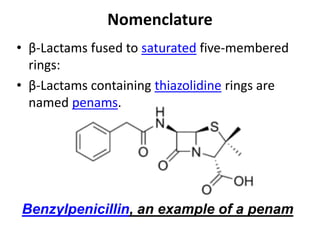
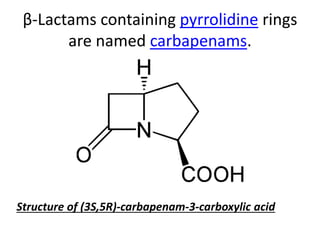
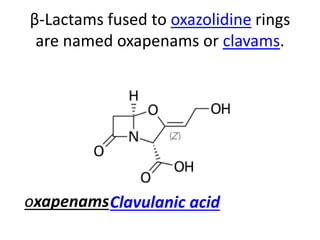
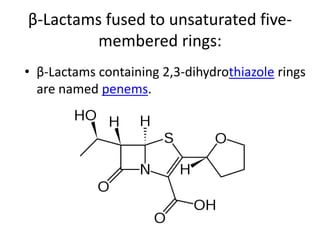
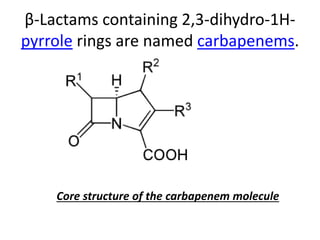
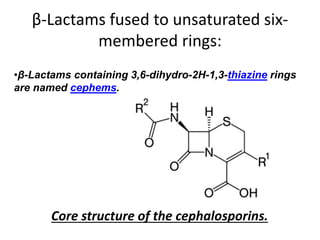
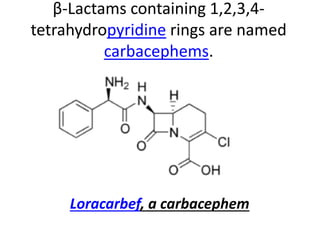
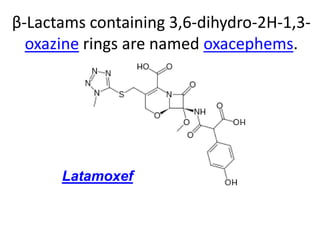
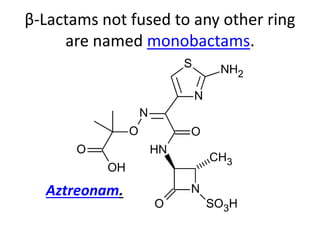
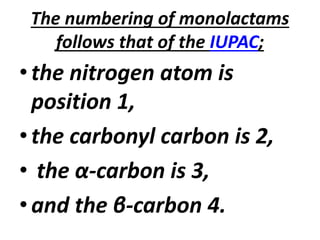
Antibiotics are drugs that kill or inhibit the growth of bacteria. They work by either killing bacteria or preventing them from growing and dividing. The most common class of antibiotics are beta-lactam antibiotics, which include penicillins, cephalosporins, monobactams, and carbapenems. Beta-lactam antibiotics work by inhibiting the synthesis of peptidoglycan in bacterial cell walls. Bacteria can develop resistance through producing beta-lactamase enzymes or by altering penicillin-binding proteins. Different classes of beta-lactam antibiotics are named based on the ring structure they are fused to.